Butylated Hydroxytoluene - Properties and Application
Chemical Properties
Butylated hydroxytoluene are also be named as 2, 6-di-tert-butyl-4-methylphenol, 2, 6-di-tert-butyl-p-cresol, Antioxidant 264, 2, 6-di-tert-butyl-p-methylphenol, BHT, DBPC, dibutylhydroxytoluene. The Molecular Weight of BHT is 220.35. As a white solid with faint characteristic odor, melting and boiling point of BHT is up to 69-73°C and 265°C respectively. At ambient condition, it is insoluble in water but soluble in ethanol (100 mg / mL) and vegetable oils. In most instances, butylated hydroxytoluene is stable, but light-sensitive, combustible and incompatible with acid chlorides, acid anhydrides, brass, copper, copper alloys, steel, bases, oxidizing agents. Butylated hydroxytoluene has wide application, such as flavors, fragrances, biochemical reagents-other chemical reagents, chemical raw materials, organic chemical raw materials, biochemical, inorganic salts, antioxidants, food additives, feed additives, feed storage additives, aromatic hydrocarbons, bulk drugs and so on. As a phenolic antioxidant, butylated hydroxytoluene can inhibit lipid peroxidation and exhibit electrophilic quinone methyl ether toxicity mediated by oxidative metabolism. The BHT metabolites, 6-tert-butyl-2- [2 ′-(2′-hydroxymethyl) -propyl] -4-methylphenol, may cause lung damage in mice and promote tumor growth.
Applications
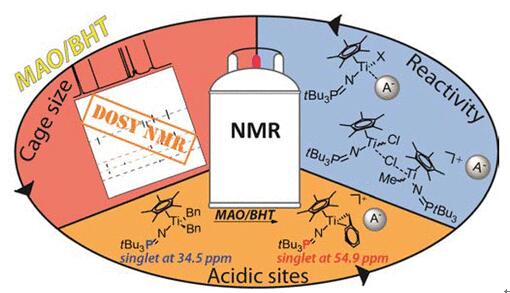
Fig 1. Butylated hydroxytoluene (BHT) used as a modified agent for catalyst
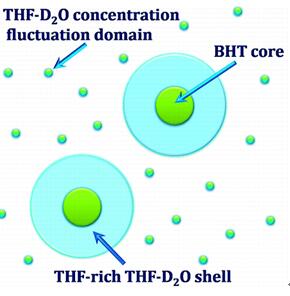
Fig 2. BHT as an antioxidant in Tetrahydrofuran–Water mixture
The applications of butylated hydroxytoluene (BHT) have been reported as following [1-9]:
• Butylated hydroxytoluene metabolites causing DNA strand breaks in cultured cells and DNA breaks between nucleosomes (a typical feature of apoptosis), which result in relieving inflammation.
• Inhibiting secretion, aggregation, and protein phosphorylation caused by protein kinase C activators at the process of the pre-incubation of aspirin-treated platelets.
• Inhibiting liver cancer formation induced by aflatoxin B1.
• As Michael receptor, butylated hydroxytoluene can react with uninucleophiles and proteins.
• Reaction of 2, 6-di-tert-butyl-4-methylphenol with fluorine (II) - benzophenone dianion complex.
• Food additive 2, 6-di-tert-butyl-4-methylphenol can promote acute lung toxicity and tumor growth in mice.
• Butylated hydroxytoluene can be used to prepare organoaluminum compound methylaluminum bis (2, 6-di-tert-butyl-4-alkylphenol oxide).
References
[1] Lydic T A, Townsend S, Adda C G, et al. Rapid and comprehensive ‘shotgun’lipidome profiling of colorectal cancer cell derived exosomes[J]. Methods, 2015, 87: 83-95.
[2] Arimura G, Köpke S, Kunert M, et al. Effects of feeding Spodoptera littoralis on lima bean leaves: IV. Diurnal and nocturnal damage differentially initiate plant volatile emission[J]. Plant Physiology, 2008, 146(3): 965-973.
[3] Martínez-Bujidos M, Rull A, González-Cura B, et al. Clustering/apolipoprotein J binds to aggregated LDL in human plasma and plays a protective role against LDL aggregation[J]. The FASEB Journal, 2015, 29(5): 1688-1700.
[4] Lewis M A, Graff Yoerg D, Bolton J L, et al. Alkylation of 2 ‘-deoxynucleosides and DNA by quinone methides derived from 2, 6-di-tert-butyl-4-methylphenol[J]. Chemical research in toxicology, 1996, 9(8): 1368-1374.
[5] Lin X, Ni Y, Kokot S. Glassy carbon electrodes modified with gold nanoparticles for the simultaneous determination of three food antioxidants[J]. Analytica chimica acta, 2013, 765: 54-62.
You may like
Related articles And Qustion
See also
Lastest Price from Butylated Hydroxytoluene manufacturers

US $0.00/Box2025-10-13
- CAS:
- 128-37-0
- Min. Order:
- 1Box
- Purity:
- 99%
- Supply Ability:
- 10tons

US $50.00-10.00/kg2025-09-02
- CAS:
- 128-37-0
- Min. Order:
- 1kg
- Purity:
- 99%,Electronic grade(Single metal impurity≤ 100ppb) or pharmaceutical grade
- Supply Ability:
- 100kg