3-Chloroperbenzoic acid (m-CPBA): A Versatile Reagent in Organic Synthesis
General Description
3-Chloroperoxybenzoic acid (mCPBA) is a peroxy acid with chemical formula C7H5ClO3. 3-Chloroperoxybenzoic acid is a strong oxidizing agent that may cause fire upon contact with flammable material. It is an example of a peroxycarboxylic acid. As a pure substance, it can be detonated by shock or by sparks. It is therefore sold commercially as a much more stable mixture that is about 75% 3-Chloroperoxybenzoic acid with the balance being 3-chlorobenzoic acid and water. 3-Chloroperoxybenzoic acid is used widely as a reagent in organic chemistry to carry out a variety of chemical transformations. It is often preferred to other peroxy acids because of its relative ease of handling. The main areas of use are the conversion of ketones to esters (Baeyer-Villiger oxidation), epoxidation of alkenes, oxidation of sulfides to sulfoxides and sulfones, and oxidation of amines to produce amine oxides.1
Intially 3-Chloroperoxybenzoic acid was has been used extensively for the determination of the total unjaturation in various types of organic compounds. In recent decades 3-Chloroperoxybenzoic acid has been used oxidation of carbonyl compounds, alcohols, iminoindolines, olefins, alkynes, carboxylic acids, amines, imines, alkanes, silyl enol ethers, N- and S-heterocycles, active methylene groups, fluoromethylated allylic bromides, cyclic acetals, ketals, diazoketones, N-substituted phthalimidines, selenides, furans, phosphates, and N-oxidation.2

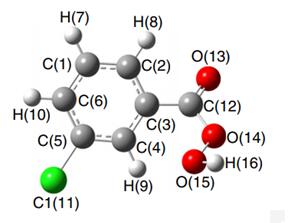
Figure 1. Properties of 3-Chloroperoxybenzoic acid
Physicochemical Proties
3-Chloroperoxybenzoic acid is a white powder (mp 90 °C), easy to handle, flammable, and hygroscopic. It is soluble in CH2Cl2, CHCl3, 1,2-dichloroethane, EtOAc, EtOH, tBuOH, Et2O, benzene and also it is slightly soluble in hexane, CCl4 and insoluble in H2O. However pure 3-Chloroperoxybenzoic acid is shock-sensitive and can deflagrate. Moreover it is potentially explosive beyond 85% purity and shows 1% degradation per year at room temperature. It is interesting to note that 85% mCPBA is not shock-sensitive and it should be stroed in a refrigerator in tightly closed containers. 3-Chloroperoxybenzoic acid irritates the mucous membranes, respiratory tract, eyes and skin and moreover skin contact with 3-Chloroperoxybenzoic acid cause in burns and blisters. Therefore it is recommended that 3-Chloroperoxybenzoic acid should be used only in a chemical fume hood.3
Applications
3-Chloroperoxybenzoic acid is a cheap commercially available oxidant that easily oxidizes numerous functional groups. It is an efficient single oxygen atom donor since it contains a non-symmetrical O–O bond which is heterolytically cleaved during the oxidation cycle. A tactical utilization of 3-Chloroperoxybenzoic acid in synthetic plans is that it may replace tedious organic transformations with simpler routes. One drawback of 3-Chloroperoxybenzoic acid is that this oxidizing regant only epoxidize electron-rich olefins and allylic or homoallylic alcohols and also 3-Chloroperoxybenzoic acid also require a directing group. One other drawback which needs to be mentioned is that a relatively large excess of 3-Chloroperoxybenzoic acid may be required in some reactions to consume all of the starting material. It is interesting to note that militating against this is that 3-Chloroperoxybenzoic acid can be reused when it is in stoichiometric excess. Owing to the discovery of a variety of novel applications, 3-Chloroperoxybenzoic acid is becoming an increasingly important reagent in synthetic organic chemistry.
Baeyer–Villiger oxidation
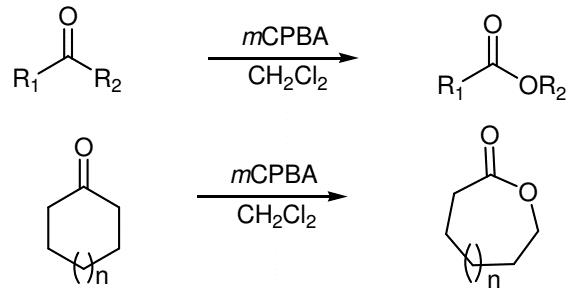
Figure 2. Baeyer–Villiger oxidation of ketones using 3-Chloroperoxybenzoic acid
The Baeyer–Villiger oxidation by the 3-Chloroperoxybenzoic acid consists of the nucleophilic addition of the 3-Chloroperoxybenzoic acid reagent to the carbonyl carbon of the substrate to afford the tetrahedral Criegee intermediate. The intermediate undergoes intramolecular rearrangement of an alkyl or aryl substituent from the central carbon to the adjacent oxygen and this migration is accompanied by cleavage of the weaker O-O bond and simultaneous formation of the ester (or lactone) and a carboxylic acid.4
Meisenheimer rearrangement
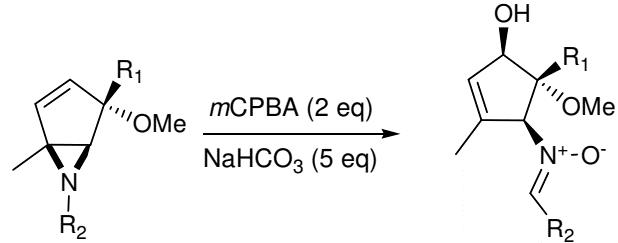
Figure 3. Meisenheimer rearrangement using 3-Chloroperoxybenzoic acid
Penkett and Simpson reported the oxidation of a range of aziridines with 3-Chloroperoxybenzoic acid whichgave products arising from a [2,3] Meisenheimer rearrangement of the initial N-oxide, followed by further oxidation to give nitrones. It was found that two equivalents of mCPBA were required. It was further found that the reaction gave low yields of product in CH2Cl2 and that the yield improved considerably when the reaction was carried out in methanol or acetonitrile. For good selectivity to be observed, it is necessary for oxidation of the aziridines to be faster than the oxidation of the nitrones. The initial oxidation step is likely to involve a larger increase in dipole moment than the second and thus a polar solvent such as acetonitrile would be expected to improve the selectivity for the nitrones. Methanol is also a very polar solvent. However the aziridinyl nitrogen lone pair of aziridines would to an extent be deactivated towards oxidation by hydrogen bonding to the solvent. This would reduce the rate of the initial oxidation and perhaps account for the reversal of selectivity. It seems likely that the reaction proceeds via the oxidation of the aziridines give the N-oxide, which undergoes rapid Meisenheimer rearrangement followed by further oxidation of the nucleophilic nitrogen, and then base catalysed N-O bond cleavage gives the nitrones.5
Cope-elimination
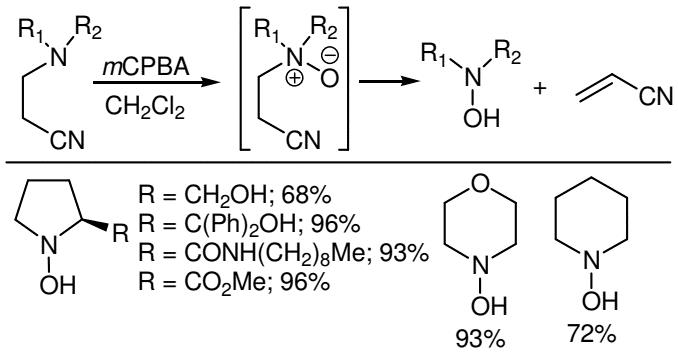
Figure 4. Cope-elimination using 3-Chloroperoxybenzoic acid
Initially Nagasawa et al. reported that the use of an appropriately positioned β-electron withdrawing nitrile group allows the Cope-elimination to occur at significantly lower temperatures than usually required. In another study O'Neil et al. reported the oxidation of a range of β-cyanoethyl tertiary amines with 3-Chloroperoxybenzoic acid to afford the corresponding Noxides, which can either be isolated or allowed to undergo Cope-elimination to give secondary hydroxylamines. The reaction works for both cyclic and acyclic systems.6
Rubottom oxidation
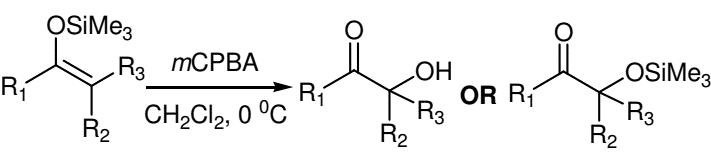
Figure 5. Rubottom oxidation using 3-Chloroperoxybenzoic acid
The synthesis of α-hydroxy ketones is achieved by reaction of silyl enol ethers with 3-Chloroperoxybenzoic acid, with subsequent rearrangement. Aqueous workup gives the desired product after desilylation. Silyl enol ethers are readily prepared from enolizable ketones using base and chlorosilane. The silyl enol ethers are usually treated with a slight excess of 3-Chloroperoxybenzoic acid in CH2Cl2 at 0 ºC followed by workup by addition of pentane. Perusal of Figure 5, which summarizes several representative Rubottom oxidation products, reveals that the introduction of the hydroxy group is regiospecific and that no exchange occurs with respect to the position of the original carbonyl group in the ketonic precursor. However, nonaqueous workup of the oxidation products yielded α- trimethylsiloxy ketones.7
Preparation
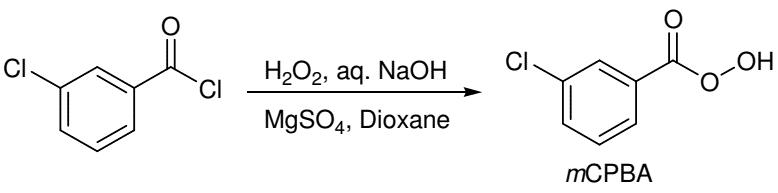
Figure 6. Preparation of 3-Chloroperoxybenzoic acid
3-Chloroperoxybenzoic acid can be prepared by the reaction of 3-chlorobenzoyl chloride with H2O2 in presence of MgSO4.7H2O, aqueous NaOH and dioxane.8
Handling and Safety
3-chlorobenzoyl chloride has been frequently employed over the years with many examples of its use on pilot scale and pharmaceutical manufacturing. However, the safety concerns with its use on scale-up are also well-known, with the pure solid being shock-sensitive and potentially explosive in the condensed phase. The commercial grade (70-77 wt %) although somewhat stabilised with chlorobenzoic acid and water still represents a significant concern when used on scale. It has been reported that CH2Cl2 could be a safer solvent for preparation of 3-chlorobenzoyl chloride solution, but recently it has been reported that that that CH2Cl2 cannot be viewed as an inherently safer solvent for preparation of 3-chlorobenzoyl chloride solutions at high concentration (large scale). However on implementation of some safety measures 3-chlorobenzoyl chloride/DMF solutions could successfully applied at large. 3-chlorobenzoyl chloride is a white powder and soluble in CH2Cl2, CHCl3, 1,2-dichloroethane, ethylacetate, benzene, and ether. However 3-chlorobenzoyl chloride is slightly soluble in hexane and insoluble in water.
References
1. E. Gipstein, F. Nichik, J. A. Offenbach, Anal. Chim. Acta, 1968, 43, 129.
2. R. Tank, Synlett 2007, 664.
3. X. Zhang, A. Hu, C. Pan, Q. Zhao, X. Wang, J. Lu, Org. Process Res. Dev. 2013, 17, 1591.
4. S. Yamabe and S. Yamazaki, J. Org. Chem., 2007, 72, 3031.
5. C. S. Penkett and I. D. Simpson, Tetrahedron Lett., 2001, 42, 3029.
6. I. A. O'Neil, E. Cleator and D. J. Tapolczay, Tetrahedron Lett., 2001, 42, 8247.
7. C.R. Krueger and E. G. Rochow, J. Organometal. Chem., 1964, 1, 476.
8. R. N. McDonald, R. N. Steppel, J. E. Dorsey, Organic Syntheses, Coll. Vol. 6, p.276 (1988); Vol. 50, p.15 (1970).
You may like
Related articles And Qustion
See also
Lastest Price from 3-Chloroperoxybenzoic acid manufacturers

US $9.90/KG2025-04-21
- CAS:
- 937-14-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5tons

US $30.00-10.00/kg2024-10-11
- CAS:
- 937-14-4
- Min. Order:
- 10kg
- Purity:
- 99%
- Supply Ability:
- 100000kg