Methyl anthranilate: production and applications
General Description
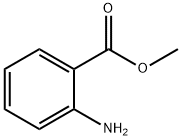
Methyl anthranilate (MANT) is a natural metabolite that gives the characteristic odor of Concord grapes and several essential oils. However, directly extracting MANT from these plants is challenging due to low yields. Currently, MANT is mostly commercially manufactured through petroleum-based chemical processes, but this method has several disadvantages, including toxic waste and labeling the resulting product as "artificial flavor". Alternatively, optimizing key enzyme expression, increasing precursor supply, and enhancing cofactor availability and salvage in microbial production have enabled the direct fermentative production of MANT from glucose. This approach has shown potential for commercial production of MANT. MANT also exhibits several potential applications, including as an antifungal agent against various fungi and a bacterial culture. In addition, studies have shown that MANT can interfere with quorum sensing and act as an anti-biofilm agent in common aquaculture pathogens and improve food safety. Furthermore, MANT has repellent effects on certain pests, such as western corn rootworm larvae, and may be effective in push-pull control strategies. Overall, the development of microbial production and the identification of MANT's potential applications offer promising opportunities for sustainable and natural MANT production and use.

Figure 1. Methyl anthranilate
Production
Extract from natural products
MANT is a natural metabolite giving the characteristic odor of Concord grapes and occurs also in several essential oils (e.g., neroli, ylang ylang, and jasmine) But it has been challenging and economically infeasible to directly extract MANT from these plants due to low yields. 1
Petroleum-based production
Currently, MANT is commercially manufactured by petroleum-based chemical processes, which mainly rely on esterification of anthranilic acid with methanol or isatoic anhydride with methanol, using homogeneous acids as catalysts. These processes, however, suffer from several disadvantages, for example, the requirement of acid catalysts in large quantities and problems with disposal of these toxic liquid acids after the reaction Moreover, MANT produced by such chemical methods is labeled “artificial flavor,” which does not meet the increasing demand by consumers for natural flavors. 2
Microbial production
Metabolic engineering of synthetic plant-derived pathways in Escherichia coli and Corynebacterium glutamicum strains has enabled the direct fermentative production of MANT from glucose. This involves optimizing key enzyme expression, increasing direct precursor supply, and enhancing cofactor availability and salvage, resulting in improved MANT production in both microorganisms. To overcome MANT toxicity, an in situ two-phase extractive fermentation using tributyrin as an extractant was developed. Using fed-batch cultures of final engineered E. coli and C. glutamicum strains, the two-phase cultivation mode produced 4.47 and 5.74 g/L MANT, respectively, in minimal media containing glucose. These metabolic engineering strategies have potential for producing other volatile aromatic esters, including MANT. 3
Applications
Antifungal agent
MANT is a fruity volatile compound found in strawberries, contributing to its grape-like aroma. MANT has been shown to have significant antifungal activity against Botrytis cinerea, Colletotrichum gloeosporioides, Colletotrichum acutatum, Phomopsis obscurans, and Gnomonia fragariae at concentrations of 1 mM, with complete cessation of fungal growth at 5 mM. Additionally, 1 mM of MANT inhibited the growth of Phytophthora cactorum. It also exhibited bacteriostatic effects in Xanthomonas cultures. The study further showed that post-harvest infestations on store-bought strawberries were inhibited by volatile treatment containing MANT. These findings demonstrate potential applications of MANT as an antifungal agent and suggest its involvement in enhancing shelf life and inhibiting premature germination. 4
Quorum sensing inhibitor and anti-biofilm agent
MANT has been studied for its potential to interfere with the quorum sensing (QS) system in Aeromonas sobria, a common aquaculture pathogen that regulates virulence potential and biofilm formation. At sub-Minimum Inhibitory Concentrations (subMICs), 0.5 μL/mL of MANT significantly reduced the formation of biofilm (by 51.44%), swinging motility (by 74.86%), swarming motility (by 71.63%), protease activity (by 43.08%), and acyl-homoserine lactone production in A. sobria. Real-time quantitative PCR and in silico analysis suggested that MANT may inhibit the QS system in A. sobria by interfering with the biosynthesis of AHL and competitively binding with receptor protein. The findings indicate the potential of MANT as a promising QS inhibitor and anti-biofilm agent for improving food safety. 5
Repellent
Methyl anthranilate exhibit repellent effects on neonate western corn rootworm larvae. A bioassay-driven approach was used to isolate the active material from diethyl ether extracts of germinating maize seed roots. Two compounds, indole and methyl anthranilate, were detected in the active fraction obtained from Florisil column separation. Methyl anthranilate yielded a significant (P < 0.05) repellent response in behavioral bioassays at doses of 1, 10, and 100 mg, preventing larvae from approaching carbon dioxide concentrations normally attractive to them. In contrast, indole did not elicit a behavioral response. These findings suggest that methyl anthranilate has potential as a management tool for western corn rootworm larvae and may be effective in push-pull control strategies. 6
UV Protection
Methyl anthranilate can be used in UV-protective sunscreen gels through a microwave-assisted method to prepare methyl-anthranilate-loaded silver nanoparticles (MA-AgNPs), which showed improved antioxidant activity and a sun protection factor of 35.75; the developed MA-AgNPs formulation was converted into a gel, demonstrating non-Newtonian pseudoplastic behavior typical in skin-care products, and was found to be stable during stability studies and effective in efficiently delivering the active ingredient to deeper layers of the skin for increased efficacy in treating skin conditions. 7
Reference
1. Wang J, De Luca V. The biosynthesis and regulation of biosynthesis of Concord grape fruit esters, including "foxy" methylanthranilate. Plant J, 2005, 44:606-619.
2. Yadav GD, Krishnan MS. An ecofriendly catalytic route for the preparation of perfumery grade methyl anthranilate from anthranilic acid and methanol. Org Process Res Dev, 1998, 2:86-95.
3. Luo ZW, Cho JS, Lee SY. Microbial production of methyl anthranilate, a grape flavor compound. Proc Natl Acad Sci U S A, 2019, 116(22):10749-10756.
4. Chambers AH, Evans SA, Folta KM. Methyl anthranilate and γ-decalactone inhibit strawberry pathogen growth and achene Germination. J Agric Food Chem, 2013, 61(51):12625-12633.
5. Li T, Sun X, Chen H, et al. Methyl anthranilate: A novel quorum sensing inhibitor and anti-biofilm agent against Aeromonas sobria. Food Microbiol, 2020, 86:103356.
6. Bernklau EJ, Hibbard BE, Norton AP, Bjostad LB. Methyl Anthranilate as a Repellent for Western Corn Rootworm Larvae (Coleoptera: Chrysomelidae). J Econ Entomol, 2016, 109(4):1683-1690.
7. Ghazwani M, Hani U, Alqarni MH, Alam A. Development and Characterization of Methyl-Anthranilate-Loaded Silver Nanoparticles: A Phytocosmetic Sunscreen Gel for UV Protection. Pharmaceutics, 2023, 15(5):1434.
Related articles And Qustion
See also
Lastest Price from Methyl anthranilate manufacturers

US $0.00/KG2025-04-21
- CAS:
- 134-20-3
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $6.00/kg2025-04-21
- CAS:
- 134-20-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month