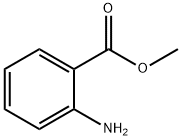
What is Methyl anthranilate?
Methyl anthranilate, also known as MA, methyl 2-aminobenzoate, or carbomethoxyaniline, is an ester of anthranilic acid manufactured through chemical synthesis, available as clear to pale yellow liquid (graph 1). Its chemical formula is C8H9NO2. It has a fruity grape smell, and one of its key uses is as a flavoring agent [1]. Methyl anthranilate acts as a bird repellent and it is food-grade and can be used to protect corn, sunflowers, rice, fruit, and golf courses. It is also used for the flavor of grape Kool-Aid and it is used for flavoring of candy, soft drinks (e.g. grape soda), fruit, chewing gum, drugs, and nicotine products. Currently, no significant side effects reported about methyl anthranilate.
Graph 1 The appearance of methyl anthranilate
Methyl anthranilate both as a component of various natural essential oils and as a synthesised aroma-chemical is used extensively in modern perfumery. It is also used to produce Schiff bases with aldehydes, many of which are also used in perfumery. In a perfumery context the most common Schiff's Base is known as aurantiol, produced by combining methyl anthranilate and hydroxycitronellal [2].
Additionally, methyl anthranilate is widely used as intermediate in pharmaceutical. Methyl N-methylanthranilate (DMA) is a valuable compound used in the flavouring and fragrance industry and is also reported to be a bird repellent. A classical route is N-methylation of methyl anthranilate with a methylating agent as dimethyl sulfate or methyl iodide. To avoid dimethylation, reductive methylation with formaldehyde and hydrogen has been described. Reaction is shown in scheme 1 [3].
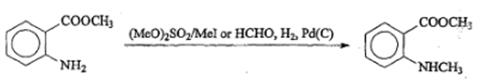
Scheme 1 Preparation of Methyl N-methylanthranilate with Methyl anthranilate
How to prepare methyl anthranilate? Esterification is a very widely understood reaction especially in the pharmaceutical, perfumery, and flavour industry. Several synthetic routes exist to make esters, but most of them are not suitable to meet the stringent specifications applied in the perfumery and flavour industry. Yadav and Mehta have reviewed these methods, including catalysis. The most acceptable method of making an ester is to react the corresponding acid with an alcohol. The reaction is catalysed by acids and is reversible. Several methods are available to drive the reaction towards the favourable product. One of them is to use an excess amount of alcohol, and the other way is to remove the ester formed (or the coproduct water) continuously. The general reaction is as following scheme 2 [4]:
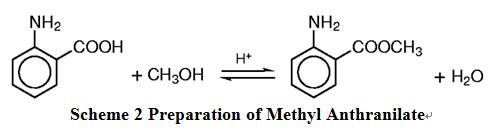
Scheme 2 Preparation of Methyl Anthranilate
References
[1] https://www.alfa.com/en/catalog/A15681/
[2] https://en.wikipedia.org/wiki/Methyl_anthranilate
[3] Carsten Berg, Process for the preparation of alkyl n-alkylanthranilate, EP1322594B1
[4] G. D. Yadav and M. S. Krishnan, An Ecofriendly Catalytic Route for the Preparation of Perfumery Grade Methyl Anthranilate from Anthranilic Acid and Methanol, Org. Proc. Res. Dev. 1998, 2, 2, 86-95
You may like
Related articles And Qustion
Lastest Price from Methyl anthranilate manufacturers

US $0.00/KG2025-04-21
- CAS:
- 134-20-3
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $6.00/kg2025-04-21
- CAS:
- 134-20-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month