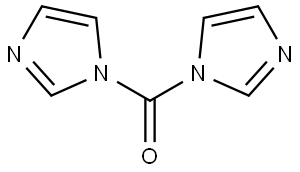
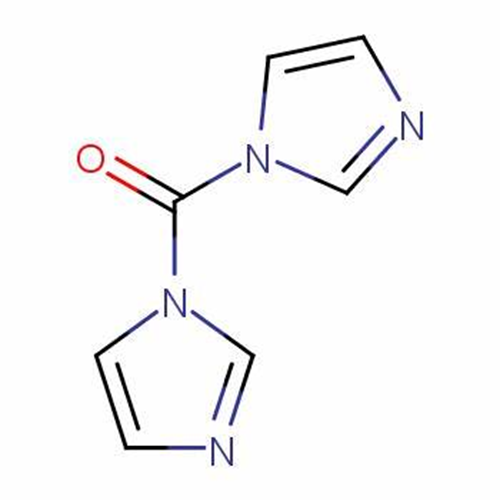
What is 1,1'-Carbonyldiimidazole?
1,1'-Carbonyldiimidazole (CDI) is an organic compound with the molecular formula (C3H3N2) 2CO. It is a white crystalline solid as shown in graph 1 and it is often used for the coupling of amino acids for peptide synthesis and as a reagent in organic synthesis [1].

Graph 1 The appearance of 1,1'-Carbonyldiimidazole
The common method of peptide synthesis is not feasible for industry use due to its poor yields. CDI, a new peptide forming reagent, is applied recently as a more direct agent for the formation of acyl-imidazoles. The peptide coupling reagent: 1,1'-carbonyldiimidazole acts as a coupling reagent and utilized for coupling of amino acids in order to prepare peptide in organic synthesis and it is also used in the preparation of beta-keto sulfones, sulfoxides and beta-enamino acid derivatives. It is used to convert alcohols and amines into carbamates, esters, and ureas. It is involved in the preparation of formylized imidazole by reaction with formic acid. Further, it is used in the synthesis of dipolar polyamides compounds. In addition to this, it is considered as an equivalent of phosgene and used to prepare asymmetric bis alkyl carbonate [2].
As shown in scheme 1, CDI can be prepared straight forwardly by the reaction of phosgene with four equivalents of imidazole under anhydrous conditions. The yield of colorless crystalline 1,1'-carbonyldiimidazole is 80–94%. The product obtained in this way sinters at 110° and melts between 112° and 117°. This material can be used without further purification for most reactions, e.g., ester, peptide, and aldehyde syntheses. The purity of a product of m.p. 113–117° was 98±2% and the quality and yield of 1,1'-carbonyldiimidazole are not reduced when the scale is doubled [3].
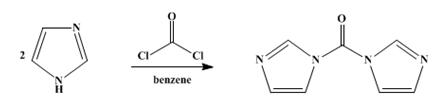
Scheme 1 Synthesis of 1,1'-Carbonyldiimidazole
It served as a convenient reagent for peptide synthesis since its side products, carbon dioxide and imidazole, were safe. Moreover, the carbon dioxide evolution was suggested to provide a driving force for the peptide synthesis. A typical peptide-formation reaction was started by dissolving 0.010 mole acylamino acid in 10 ml tetrahydrofuran (THF), and after this, the evolution of carbon dioxide could be immediately observed. An hour later, the required amino acid or peptide ester was added with an amount of 0.010 molar. The reaction would be last for 15 min, but longer standing is probably beneficial. The formed peptide was then purified by wiping off the solvent under vacuum followed by washing the residue with acid, saturated bicarbonate and finally water.
References
[1] https://en.wikipedia.org/wiki/Carbonyldiimidazole
[2] https://www.alfa.com/en/catalog/A14688/
[3] http://orgsyn.org/demo.aspx?prep=CV5P0201
Related articles And Qustion
Lastest Price from 1,1'-Carbonyldiimidazole manufacturers
US $1.00/g2025-10-22
- CAS:
- 530-62-1
- Min. Order:
- 10g
- Purity:
- 99.9%
- Supply Ability:
- 20 TONS
US $1.00/g2025-10-21
- CAS:
- 530-62-1
- Min. Order:
- 300g
- Purity:
- 99.8%
- Supply Ability:
- 20 TONS