Prilocaine: Applications and dosage
General description
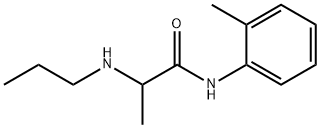
Prilocaine is a local anesthetic of the amino amide type first prepared by Claes Tegner and Nils Löfgren. In its injectable form (trade name Citanest), it is often used in dentistry. It is also often combined with lidocaine as a topical preparation for dermal anesthesia (lidocaine/prilocaine or EMLA), for treatment of conditions like paresthesia. As it has low cardiac toxicity, it is commonly used for intravenous regional anaesthesia (IVRA). In some patients, ortho-toluidine, a metabolite of prilocaine, may cause methemoglobinemia, which may be treated with methylene blue. Prilocaine may also be contraindicated in people with sickle cell anemia, anemia, or symptomatic hypoxia. [1] It is given as a combination with the vasoconstrictor epinephrine under the trade name Citanest Forte. It is used as an eutectic mixture with lidocaine, 50% w/w, as lidocaine/prilocaine. The mixture is an oil with a melting point of 18 °C (64 °F). A 5% emulsion preparation, containing 2.5% each of lidocaine/prilocaine, is marketed by APP Pharmaceuticals under the trade name EMLA (an abbreviation for eutectic mixture of local anesthetics). Its appearance is as follows:

Figure 1 Appearance of Prilocaine
Applications
Prilocaine is an amino acid amide in which N-propyl-DL-alanine and 2-methylaniline have combined to form the amide bond; used as a local anaesthetic. Prilocaine has a role as a local anaesthetic and an anticonvulsant. The lidocaine/prilocaine combination is indicated for dermal anaesthesia. Specifically it is applied to prevent pain associated with intravenous catheter insertion, blood sampling, superficial surgical procedures; and topical anaesthesia of leg ulcers for cleansing or debridement. [2] Also, it can be used to numb the skin before tattooing as well as electrolysis and laser hair removal. It is also sometimes used in advance of injected local anaesthetics for minor surgery and biopsies. A topical spray consisting of an aerosol formulation of lidocaine and prilocaine was evaluated under the name PSD502 for use in treating premature ejaculation. The spray is applied on the penile skin prior to intercourse. While this formulation was not approved by the FDA, a similar product, Promescent, is available over-the-counter in the U.S. Lidocaine/prilocaine eutectic mixture is used during circumcision in newborn boys and has been considered efficacious and safe to lessen pain from circumcision. [3] In addition, the spray is a combination of local anaesthetics lidocaine and prilocaine in a metered-dose aerosol that is sprayed directly on the penis to numb sensations. It was developed by the same group that invented the erectile dysfunction drug sildenafil. The drug was approved in Europe and was released in the UK market in November 2016 and within the EU will be marketed by, early in 2017 In the United States, the Food and Drug Administration (FDA) is expected to approve the drug in 2018. [4]
Dosage
Lidocaine/prilocaine eutectic mixture is marketed as a 5% oil-in-water emulsion incorporated in a cream base (EMLA cream) or a cellulose disk (EMLA patch). The cream is applied under an occlusive dressing, while the patch incorporates an occlusive dressing to facilitate absorption of lidocaine and prilocaine into the area where anaesthesia is required. Local dermal anaesthesia is achieved after approximately 60 minutes, whereupon the occlusive dressing (or patch) is removed. The duration of anaesthesia is approximately two hours following removal of the occlusive dressing. E. Fougera & Co., makers of the generic cream widely used in the United States as Lidocaine and Prilocaine Cream, 2.5%/2.5%, recommends different timing for application of the cream as well as length of anesthesia. They state the cream must be applied at least one hour before the start of a routine procedure and for two hours before the start of a painful procedure. Additionally, they state that the duration of effective skin anesthesia will be at least one hour after removal of the occlusive dressing. [5]
References
[1]Patel V, Morrissey J (2011-09-15). Practical and Professional Clinical Skills. Oxford University Press. p. 267. ISBN 9780199585618.
[2]Heinen MM, van Achterberg T, op Reimer WS, van de Kerkhof PC, de Laat E (March 2004). "Venous leg ulcer patients: a review of the literature on lifestyle and pain-related interventions". Journal of Clinical Nursing. 13 (3): 355–66. doi:10.1046/j.1365-2702.2003.00887.x. PMID 15009338.
[3]Taddio A, Ohlsson K, Ohlsson A (2000). Ohlsson A (ed.). "Lidocaine-prilocaine cream for analgesia during circumcision in newborn boys". The Cochrane Database of Systematic Reviews (2): CD000496. doi:10.1002/14651858.CD000496. PMID 10796371.
[4]"Viagra Inventor Develops 'Tempe Spray' To Help Men Deal With Premature Ejaculation: Is It Safe?". medicaldaily.com. Retrieved 25 September 2013.
[5]Lidocaine and Prilocaine Cream, 2.5%/2.5%, package insert. E. Fougera & Co. package insert. Melville, New York, 9/2007.
You may like
Related articles And Qustion
Lastest Price from Prilocaine manufacturers

US $5.00-0.50/KG2025-06-05
- CAS:
- 721-50-6
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $5.00-0.50/KG2025-05-07
- CAS:
- 721-50-6
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS