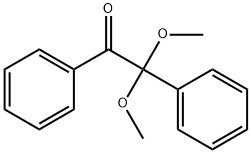
2,2-Dimethoxy-2-phenylacetophenone: an efficient radical photoinitiator
2,2-Dimethoxy-2-phenylacetophenone (DMPA) is a very efficient and frequently used free radical photoinitiator for thin photocurable coatings (∼ 20 μm).10, 11 The colorless nature of DMPA makes it very attractive for the formulation of dental resins with improved esthetical properties.

Preparation of 2,2-Dimethoxy-2-phenylacetophenone
Through the metering pump to dimethyl sulfate and solvent dioxane into the synthesis kettle, At the same time by adding a certain amount of output from the step-out of benzoyl, After cooling to 10 into the sodium methoxide. After the investment heated to 25, Insulation reaction 10 ~ 12h, After the end point was measured, the reaction solution was poured into an alkali kettle. The alkali kettle reaction solution was heated to about 105 ° C, Steamed dioxane for recovery (about 4h) applied; bottom liquid added liquid caustic soda, Heating up to 102 for alkali melting, reaction for about 6h, pH control at 10 or more. After the completion of water holding 70 for alkaline crystal layer, the crystal layer in the alkali layer under the base. The separated lye layer is stored by a temporary storage tank (evaporated by a three-effect evaporator and then carbonized by a carbonization furnace, The resulting sodium sulfate sold), Crystal layer and water cooling into the crystal, and then into the centrifuge dehydration, and then washed. The crude wet product 2,2-Dimethoxy-2-phenylacetophenone into the refining kettle, through the metering pump to join a certain amount of methanol (including the application of centrifugal mother liquor), heated to about 60 to dissolve, and then put a small amount of activated carbon, decolorization, After about 2.5h, the decolorization is completed. After filtering the activated carbon through the microporous filter, the filtrate is fed into the crystallization kettle to cool and crystallize. The temperature is controlled between 15 and 20. After about 3 hours, the filtrate of 2,2-Dimethoxy-2-phenylacetophenone is sent to the centrifuge for dehydration. Centrifuge the mother liquor back to the decolorization process, apply three times after the distillation column distillation. Wet boutique is sent into the double cone vacuum dryer after drying to be finished, packaged into the library. The synthesis reaction of dimethyl sulfate and sodium methoxide as the excess material, the reaction yield of 2,2-Dimethoxy-2-phenylacetophenone is about 92.6%.[1]
Analysis of poly(ethylene glycol)-diacrylate macromer polymerization
Semi-interpenetrating polymer networks (semi-IPNs) containing poly(ethylene glycol)-diacrylate (PEGdA) and modified gelatin were prepared with 2,2-Dimethoxy-2-phenylacetophenone (DMPA) as a photoinitiator. The effect of (i) initiator and PEGdA concentration, and (ii) weight ratio and type of modified gelatin on the conversion of PEGdA functional end groups was monitored in situ using attenuated total reflectance-Fourier transform infrared (ATR-FTIR). Reaction induction time was dependent on 2,2-Dimethoxy-2-phenylacetophenone concentration and increased with decreasing DMPA concentration. Relative reaction rate was strongly dependent on both 2,2-Dimethoxy-2-phenylacetophenone and PEGdA concentrations. Gelatin weight ratio and modification did not significantly affect reaction induction time, relative reaction rate, or reaction end time. Swelling/degradation kinetics at various aqueous conditions sought to establish relationships between diacrylate conversion and the resulting semi-IPN physical properties. Semi-IPN swelling weight ratio was strongly dependent on solvent conditions and semi-IPN exposure to γ-irradiation. Gelatin backbone modification and UV exposure time exhibited no effect on semi-IPN swelling weight ratio. In conclusion, ATR-FTIR presents a viable means of monitoring the conversion of PEGdA functional end groups within a complex mixture. UV exposure >10 s did not significantly affect the weight swelling ratio, and supports our ATR-FTIR results that network formation reached completion before 3 min of UV exposure.[2]
In summary, the significant effects (at p < 0.05) of PEGdA concentration, 2,2-Dimethoxy-2-phenylacetophenone concentration, and gelatin weight ratio are presented. Tind was only dependent on 2,2-Dimethoxy-2-phenylacetophenone concentration, whereas Slope and Tend were dependent on both 2,2-Dimethoxy-2-phenylacetophenone and PEGdA concentration. Slope and %Conversion were also significantly decreased when the gelatin weight ratio was reduced to zero (0G-10P). Gelatin weight ratio and modification did not significantly affect reaction induction time, reaction rate, or reaction end time.
Photoinitiators enhanced 1,2-dichloropropane
Dichloromethane (DCM) and 1,2-dichloropsropane (DCP) have various uses, including being solvents for paint removers. Photoinitiators are also used in a wide range of commercial applications such as printing. We showed that DCP, 2,2-dimethoxy-2-phenylacetophenone (2,2-Dimethoxy-2-phenylacetophenone), 2-ethylhexyl-4-(dimethylamino) benzoate (2-EHDAB), 1-hydroxycyclohexyl phenyl ketone (1-HCHPK), and methyl 2-benzoylbenzoate (MBB) induced cytotoxicity, whereas DCM and 2-isopropylthioxanthone (2-ITX) did not.[3]
In the present study, we confirmed that acute exposure to DCP induced cytotoxicity; however, acute exposure to DCM was not cytotoxic in normal human embryonic lung fibroblasts. In addition, acute exposure to 2,2-Dimethoxy-2-phenylacetophenone, 2-EHDAB, 1-HCHPK, MBB, and MTMP induced cytotoxicity in human normal embryonic lung fibroblasts, whereas 2-ITX did not. The combination of DCP and 2,2-Dimethoxy-2-phenylacetophenone, 1-HCHPK, MBB, or MTMP led to more potent cytotoxic effects and induced apoptosis in MRC-5 cells. Therefore, these results suggest that photoinitiators may play a role in lung injury due to DCP-induced cytotoxicity. The photoinitiators 2,2-Dimethoxy-2-phenylacetophenone, 2-EHDAB, 1-HCHPK, MBB, and MTMP induced cytotoxicity, whereas 2-ITX did not. Some photoinitiators have been shown to induce toxicity under several conditions.
References
[1]SHANGYU JIAYING CHEMICAL - CN104817443, 2017, B
[2]Witte RP, Blake AJ, Palmer C, Kao WJ. Analysis of poly(ethylene glycol)-diacrylate macromer polymerization within a multicomponent semi-interpenetrating polymer network system. J Biomed Mater Res A. 2004 Dec 1;71(3):508-18.
[3]Kawasaki Y, Tsuboi C, Yagi K, Morizane M, Masaoka Y, Esumi S, Kitamura Y, Sendo T. Photoinitiators enhanced 1,2-dichloropropane-induced cytotoxicity in human normal embryonic lung fibroblasts cells in vitro. Environ Sci Pollut Res Int. 2015 Mar;22(6):4763-70.
Related articles And Qustion
See also
Lastest Price from 2,2-Dimethoxy-2-phenylacetophenone manufacturers

US $1.00/Kg/Bag2025-04-21
- CAS:
- 24650-42-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5ton/month

US $10.00/kg2025-04-21
- CAS:
- 24650-42-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt