The viscosity of dimethyl ether
Introduction
Dimethyl ether (DME) is the simplest ether, without C-C bond. It is one of the hot research areas in energy chemistry and environmental chemistry recently[1]. DME can take the place of LPG due to the similar physicochemical property. Besides, DME can also be used as diesel fuel of high cetane number, especially with very low soot emission in the exhaust gas from diesel engine as it has no C-C bond structure. It can be readily decomposed even if being leaked into air, very safe and benign to the environment. DME is not only used as a final product but also employed to be converted to a lot of chemicals and energy products. With very lower poison property, DME often plays an alternative role of methanol. DME can be converted into light olefins and aromatics with zeolite catalyst. It also serves as a hydrogen source for fuel cell. Acting as a hydrogen carrier, it can be reformed again to syngas (CO+H2) or hydrogen if necessary. In some cases, DME can be oligomerized to dimethoxymathane (CH3OCH2OCH3, DMM) or polyoxymethylene dimethyl ethers (DMMn), which are liquid diesel fuels under room temperature and atmospheric pressure. Furthermore, DME is a key product or intermediate in C1 chemistry or methane/syngas chemistry. It can be catalytically transformed to methyl acetate, formaldehyde, and ethanol, via reaction with CO or syngas (a mixture of CO and H2).

Picture 1 Dimethyl ether powders
The viscosity of dimethyl ether
Dimethyl ether (DME) has been recognised as an excellent fuel for diesel engines for over one decade now. Engines fuelled by DME emit virtually no particulate matter even at low NOx levels. This is only possible in the case of diesel oil operation if expensive and efficient lowering particles and NOx traps are installed[2].
The most significant problem encountered when engines are fuelled with DME is that the injection equipment breaks down prematurely due to extensive wear. This tribology issue can be explained by the very low lubricity and viscosity of DME. Recently, laboratory methods have appeared capable of measuring these properties of DME. The development of this is rendered difficult because DME has to be pressurised to remain in the liquid state and it dissolves most of the commercially available elastomers.
Dimethyl ether (DME) as an alternative fuel
With ever growing concerns on environmental pollution, energy security, and future oil supplies, the global community is seeking non-petroleum based alternative fuels, along with more advanced energy technologies (e.g., fuel cells) to increase the efficiency of energy use. The most promising alternative fuel will be the fuel that has the greatest impact on society. The major impact areas include well-to-wheel greenhouse gas emissions, non-petroleum feed stocks, well-to-wheel efficiencies, fuel versatility, infrastructure, availability, economics, and safety. Compared to some of the other leading alternative fuel candidates (i.e., methane, methanol, ethanol, and Fischer–Tropsch fuels), dimethyl ether appears to have the largest potential impact on society, and should be considered as the fuel of choice for eliminating the dependency on petroleum[3].
DME can be used as a clean high-efficiency compression ignition fuel with reduced NOx, SOx, and particulate matter, it can be efficiently reformed to hydrogen at low temperatures, and does not have large issues with toxicity, production, infrastructure, and transportation as do various other fuels. The literature relevant to DME use is reviewed and summarized to demonstrate the viability of DME as an alternative fuel.
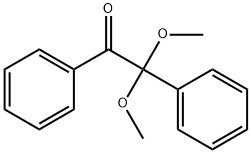
Benzoin dimethyl ether synthesis process
The present invention discloses a benzoin dimethyl ether synthesis process, which comprises[4]: adding methanol, benzaldehyde, sodium cyanide and water to a reaction kettle, carrying out a condensation reaction to generate an intermediate benzoin, adding the benzoin to an oxidation kettle, carrying out an oxidation reaction to obtain dibenzoyl, adding the dibenzoyl to a synthesis kettle, adding dimethyl sulfate and solvents such as dioxane and sodium methoxide, carrying out alkali fusion, adding water after the alkali fusion, carrying out thermal insulation at a temperature of 70 DEG C, carrying out alkali crystal layering, adding water to the crystal layer, carrying out cooling crystal transition, placing into a centrifuge to dewater, carrying out water washing centrifugation to obtain a crude wet product, pouring the crude wet product into a refining kettle, adding methanol through a metering pump, heating to a temperature of 60 DEG C to dissolve, adding active carbon, carrying out decolorization, filtering after the decolorization so as to remove the active carbon, conveying the filtrate into an crystallization kettle, carrying out cooling crystallization for 3 h to obtain a wet fine product, drying the wet fine product through filtration, washing and drying three-in-one equipment to obtain the finished product, and carrying out packaging warehousing. With the synthesis process of the present invention, the effects of low raw material cost, low environmental pollution, and high finished product yield are achieved.
Reference
1 Sun, J., Yang, G., Yoneyama, Y., & Tsubaki, N. (2014). Catalysis Chemistry of Dimethyl Ether Synthesis. ACS Catalysis, 4(10), 3346–3356.
2 Ion M.Sivebaek, The viscosity of dimethyl ether, Tribology International, Volume 40, Issue 4, April 2007, Pages 652-658
3 Troy A.Semelsberger, Dimethyl ether (DME) as an alternative fuel, Journal of Power Sources
Volume 156, Issue 2, 1 June 2006, Pages 497-511.
4 WANG XINGBING, CN104817443A;CN104817443B
Related articles And Qustion
See also
Lastest Price from 2,2-Dimethoxy-2-phenylacetophenone manufacturers

US $1.00/Kg/Bag2025-04-21
- CAS:
- 24650-42-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5ton/month

US $10.00/kg2025-04-21
- CAS:
- 24650-42-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt