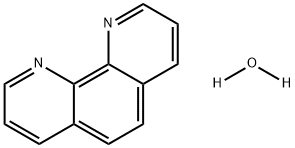
The chemical properties of 1,10-Phenanthroline hydrate
Introduction
1,10-Phenanthroline hydrate purified by recrystallization from water and dried for several hours in a desiccator. A portion of the hydrate so obtained was converted to the anhydrous compouad by fusion. The melting points are: hydrate 99-100, an- hydrous 117.
1,10-Phenanthroline hydrate (1,10-phen) is well known as a bidentate ligand in coordination chemistry. Many metal complexes involving 1,10-phen as a ligand have special properties and in order to obtain a better understanding of structures of the transition metal to 1,10-phen chelating systems, many authors have investigated the crystal structures of 1,10-phen and 1,10-phen.H20 (Donnay, Donnay & Harding, 1965; Sen, 1974; Nishigaki, Yoshioka & Nakatsu, 1975, 1978). However, no detailed structure determination of o-phen.H20 has been reported. Moreover, it is of interest to compare the crystal and molecular structure of 1,10-phen.H20 with that of 1,10-phen and to study the effect of hydrogen bonding on the crystal structure[1].

Picture 1 1,10-Phenanthroline hydrate powders
Structure
The X-ray analysis revealed that the title compound is an infinite screw-chain structure with a repeat distance of 0.8453 nm. The O atoms of the water connect to one another around the screw axis to form the core of the chain and the o-phenanthroline molecules connect through intermolecular hydrogen bonds to these water molecules. The structure can be regarded as a screw supermolecule assembled by hydrogen bonds and is a displacive modulation of an idealized P3121 parent structure[2].
Application of 1,10-Phenanthroline hydrate
Approximately 0.1 g samples were decomposed by boiling water and the metal estimated by complexometric titration with EDTA using alizarin red as indicator. The chloride or thiocyanate in another similar sample was estimated by the Volhard method. The validity of these methods in presence of 1,10-phenanthroline hydrate was confirmed by estimation of artificial mixtures of lanthanide salts and 1,10-phenanthroline hydrate of known concentration. It is however important that the Volhard estimation is performed at an acid concentration not greater than 0"5 N. The total 1,10-phenanthroline hydrate content was in a few cases estimated by direct titration against N/80 hydrochloric acid using bromophenol blue indicator; the validity of this method was similarly checked by estimation of solutions of known 1,10-phenanthroline hydrate concentration containing lanthanide salts.
The chemical properties of 1,10-Phenanthroline hydrate
The reaction for which the thermodynamic quantities have been measured is the removal of a molecule of water from a molecule of 1,10-phenanthroline monohydrate, two hydrogen bonds being broken in the process. Accordingly values of 14.5, 13.0 and 13.2 cal./mole were obtained for AH of the hydrates of 1,10-phenanthroline, the 5-bromo and the 5-methyl derivatives, respectively[3]. Since the nitrogen atoms in 1,10-phenanthroline are equivalent, the value of "/2, 7.25 cal./mole, may be considered as representing the strength of one hydrogen bond.1° The presence of a substituent in the 5 position would be expected to affect the electron density around the two ring nitrogen atoms unequally. Therefore, it cannot be assumed that AH12 in these cases represents the strength of a single hydrogen bond but that it is rather the arithmetic average of the two unequal hydrogen bonds. Compared to the 0-H```0 bonds measured by other worker, the 0-H```N bonds measured in the present work are quite strong. It was desired to study further the effect of substituents on the strengths of the hydrogen bonds by measuring the dissociation pressures of some symmetrically 1,10-phenanthrolines. Upon searching for compounds of this class only the 5,6-dimethyl derivative was available, but contrary to expectations no hydrate could be obtained. Perhaps this is due to the extraordinarily high melting point of this compound (272° compared to 114° for the anhydrous 5-methyl compound and 117° for anhydrous 1,10-phenanthroline).
Reference
1 Tian Y P, Duan C Y, Xu X X, et al. Screw-chain structure of 1, 10-phenanthroline hydrate, C12H8N2. H2O[J]. Acta Crystallographica Section C: Crystal Structure Communications, 1995, 51(11): 2309-2312.
2 Hart F A, Laming F P. Complexes of 1, 10-phenanthroline with lanthanide chlorides and thiocyanates[J]. Journal of Inorganic and Nuclear Chemistry, 1964, 26(4): 579-585.
3 Fritz J S, Cagle F W, Smith G F. The Determination of the Decomposition Pressures of Certain 1, 10-Phenanthroline Hydrates[J]. Journal of the American Chemical Society, 1949, 71(7): 2480-2482.
Related articles And Qustion
See also
Lastest Price from 1,10-Phenanthroline hydrate manufacturers

US $999.00-666.00/ton2025-04-21
- CAS:
- 5144-89-8
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 5000

US $0.00-0.00/KG2025-04-15
- CAS:
- 5144-89-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg