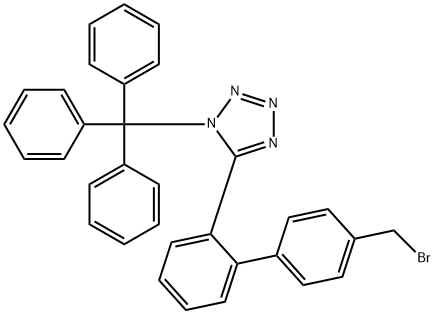
Genotoxic of N-(triphenylmethyl)-5-(4'-bromomethylbiphenyl-2-yl)tetrazolium
Introduction
N-(triphenylmethyl)-5-(4'-bromomethylbiphenyl-2-yl)tetrazolium, is a chemical substance with the molecular formula C33H25BrN4, which is used as an intermediate for the drug irbesartan. The chemical is hazardous to water bodies and should be prevented from contacting ground water, water courses or sewage systems, and material should not be released into the surrounding environment without government permission[1].
Some basic information of N-(triphenylmethyl)-5-(4'-bromomethylbiphenyl-2-yl)tetrazolium is showed as followed. The CAS number is 124750-51-2. The density is 1.282 g/cm3. Melting point is 152-155°C. Boiling point is 718.441°C at 760 mmHg. The flash point is 388.299°C. Vapor pressure is 0mmHg at 25°C. The refractive index is 1.66. Storage conditions are Inert atmosphere, 2-8°C. N-(triphenylmethyl)-5-(4'-bromomethylbiphenyl-2-yl)tetrazolium can be used as intermediate of drug irbesartan.
Properties of N-(triphenylmethyl)-5-(4'-bromomethylbiphenyl-2-yl)tetrazolium
N-(triphenylmethyl)-5-(4'-bromomethylbiphenyl-2-yl)tetrazolium (impurity 1) was used as the starting material for nitrogen alkylation and salt formation. However, incompletely reacted starting material impurity 1 and N-trityl-5-(4',4'-dibromomethylbiphenyl-2-yl)tetrazolium by-product ( Impurity 2) is likely to be passed on to the finished product, and both impurity 1 and impurity 2 have genotoxicity warning structures. However, complete exclusion of genotoxic impurities in the drug substance synthesis process is often not achievable[2].
Application of N-(triphenylmethyl)-5-(4'-bromomethylbiphenyl-2-yl)tetrazolium
Using N-(triphenylmethyl)-5-(4'-bromomethylbiphenyl-2-yl)tetrazolium as raw material, valsartan was prepared by three-step reaction of coupling, nucleophilic substitution and deprotection , the total yield is 49.6%. The process route is short, the operation is simple, the post-processing is easy, and the by-products are few. Angiotensin II receptor antagonists and non-peptide angiotensin II receptor antagonists play a very important role in the treatment of hypertension, and the representative drug is losartan[3].
Picture 1 losartan
Losartan is an oral selective hemotensin II receptor antagonist used in the treatment of hypertension. Its potassium salt, losartan potassium, was first discovered by DuPont-Merck and named as Kesuya. The drug has the advantages of good tolerance and high receptor selectivity. Losartan is a new derivative of biphenyltetrazole containing s-triazole. The structure-activity relationship and synthesis of losartan were analyzed, and it was found that 2-N-(triphenylmethyl)-5-[(4'-bromomethyl)-biphenyl-2-]tetrazolium It is an indispensable raw material for the synthesis of losartan. The synthesis and biological activity of this new derivative of biphenyltetrazole containing s-triazole is achieved by substituting triazolethione and 2-N-(triphenylmethyl)- Condensation of 5-[(4'-Bromomethyl)-biphenyl-2-]tetrazolium in K<,2>CO<,3>/CH<,3>COCH<,3> System , 14 new S-alkylated products were obtained, and the S-alkylated products were deprotected under acidic conditions to obtain 14 new biphenyltetrazole derivatives. By elemental analysis, IR, NMR and FAB-MS The structures were determined and their spectral properties were discussed. Their antibacterial activity and antihypertensive activity were preliminarily evaluated
Genotoxic of N-(triphenylmethyl)-5-(4'-bromomethylbiphenyl-2-yl)tetrazolium
Olmesartan medoxomil is a prodrug, which is rapidly and completely hydrolyzed into the active substance olmesartan in the gastrointestinal tract after oral administration and exerts a hypotensive effect[4]. Olmesartan is a selective angiotensin II type 1 receptor (AT1) antagonist, which blocks the vasoconstrictive effect of angiotensin II by selectively blocking the binding of angiotensin II to vascular smooth muscle AT1 receptors. for the treatment of hypertension. Olmesartan medoxomil is a long-term medication, and research and control of genotoxic impurities are required based on regulatory requirements. N-(triphenylmethyl)-5-(4'-bromomethylbiphenyl-2-yl)tetrazolium (BBTT1), an important starting material of olmesartan medoxomil (OLM), contains about 1% to 2 % of the dibromo impurity N-(triphenylmethyl)-5-(4'-dibromomethyl biphenyl-2-yl) tetrazolium (BBTT2), the trityl group will be removed in the reaction The protecting groups generate 5-(4'-bromomethylbiphenyl-2-yl)tetrazolium (BBT1) and 5-(4'-dibromomethylbiphenyl-2-yl)tetrazolium (BBT2), respectively , the structural formulas of the two products are shown in Figure 1, both of which are potential genotoxic impurities, which are easily ignored in the study of impurities, and there is a great quality risk.
Renference
1 Zhang Haiyun, Zeng Yang, Liao Xindan, et al. Determination of related substances in N-triphenylmethyl-5-(4'-bromomethylbiphenyl-2-yl)tetrazolium by HPLC[J]. West China Journal of Pharmacy, 2015, 30(1): 92-94.
2 Zhang Liguang, Liu Xuemei. Improvement of Valsartan Synthesis Process [J]. Contemporary Chemical Industry, 2015(9):2146-2147.
3 Chao Shujun. Synthesis and Biological Activity of New Derivatives of Biphenyl Tetrazole[D]. Gansu: Lanzhou University, 2004.
4 Yuan Baoyun, Chen Cheng, Li Jichao, et al. Determination of genotoxic impurities in olmesartan medoxomil by HPLC [J]. China Modern Applied Pharmacy, 2017,34(3):407-409.
See also
Lastest Price from 5-(4'-Bromomethyl-1,1'-biphenyl-2-yl)-1-triphenylmethyl-1H-tetrazole manufacturers
US $0.00/kg2025-09-02
- CAS:
- 124750-51-2
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons

US $0.00-0.00/mg2025-06-04
- CAS:
- Min. Order:
- 10mg
- Purity:
- 99%+ HPLC
- Supply Ability:
- 1000