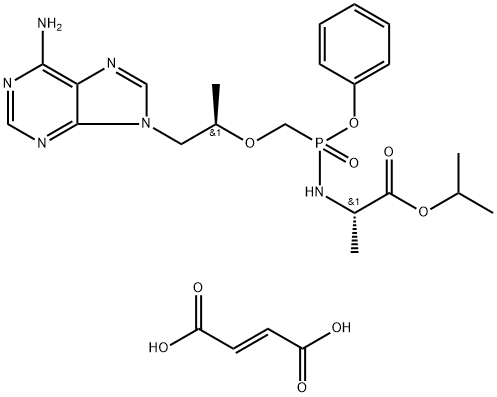
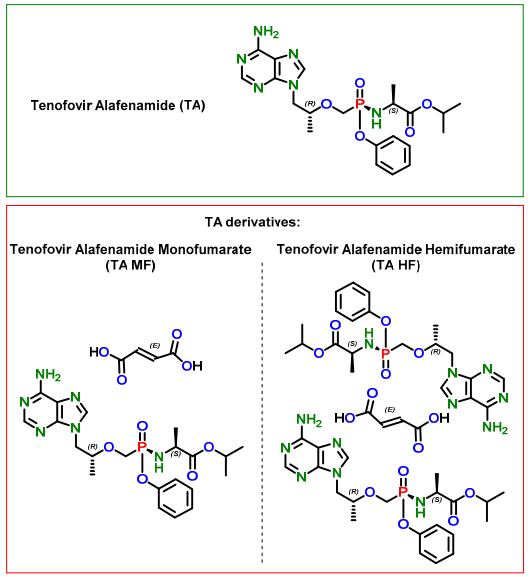
The preparation of tenofovir alafenamide fumarate
Introduction
Tenofovir alafenamide fumarate is a lipophilic prodrug of tenofovir which is preferentially metabolized in lymphatic tissue resulting in high concentrations of tenofovir (TFV) and its active diphosphate metabolite inside the cells that replicate HIV. Due to its selectivity for these tissues, lower total doses of TAF can be administered relative to tenofovir disoproxil fumarate (TDF) which results in improved bone and renal biomarkers. Tenofovir alafenamide fumarate has become the “backbone” of multiple combination products for the treatment of HIV, combined with emtricitabine for PreP and as a monotherapy for the treatment or HBV.

Picture 1 Tenofovir alafenamide fumarate tablets
Application
Tenofovir alafenamide fumarate (TAF), a new prodrug of tenofovir and a potential successor of tenofovir disoproxil fumarate (TDF), has been approved in the United States and Europe for treating adolescents and adults with chronic hepatitis B infection. TAF is formulated to deliver the active metabolite to target cells more efficiently than TDF at lower doses, thereby reducing systemic exposure to tenofovir. In patients with chronic hepatitis B, TAF appears to be as effective as TDF, with lower bone and renal toxicity. TAF has the potential advantages that dose adjustment is not required in patients with renal impairment, and monitoring can be less intense because of the better safety profile. Results from 2 large, randomized, phase 3 studies after 48 weeks of therapy have shown that TAF may be a good alternative to TDF for treating chronic hepatitis B. Whether the short-term benefits observed in these 48-week trials will translate into improvements in bone and renal health in patients receiving long-term treatment remains to be seen.
Novel preparation process of tenofovir alafenamide fumarate
The invention discloses a novel preparation process of tenofovir alafenamide fumarate, and in particular provides a preparation method of the tenofovir alafenamide fumarate. The method comprises the steps of adding a crude tenofovir alafenamide product into isobutanol, dissolving by heating, and then adding a mixed solution of acetonitrile and n-hexane for refining; then, adding the obtained tenofovir alafenamine refined product and fumaric acid into acetonitrile, salifying and crystallizing to obtain the tenofovir alafenamide fumarate. After the method provided by the invention is adopted, diastereomer impurities in crude tenofovir alafenamide product can be effectively removed, and the high-quality tenofovir alafenamide fumarate can be obtained.
Tenofovir alafenamide fumarate impurity preparing method
The invention relates to a novel synthetic method of three tenofovir alafenamide fumarate impurities. The synthetic method has important significance in synthesis of high-quality tenofovir alafenamide fumarate. The invention mainly aims at studying synthesis of a tenofovir alafenamide isopropyl ester impurity 9-[(R)-2-[[(S)-[[(S)-1-isopropoxy phenoxyl phosphinyl] methoxyl] propyl] adenine (IV), a tenofovir alafenamide diphenyl ester impurity 9-[(R)-2-[[(S)-[bi-(phenoxyl) phosphinyl] methoxyl] propyl] adenine (VI) and a tenofovir alafenamide diamide impurity 9-[(R)-2-[[bi-[[(S)-1-(isopropoxy carbonyl) ethyl] amino] phosphinyl] methoxyl] propyl] adenine (VII), and their specific synthesis routes are shown in the description.
Preparation method and application for high-purity tenofovir
The invention discloses a preparation method and an application for a high-purity tenofovir alafenamide fumarate intermediate. The preparation method for the tenofovir alafenamide fumarate intermediate namely tenofovir alafenamide II provided by the invention comprises the following steps: subjecting crude tenofovir alafenamide fumarate intermediate namely tenofovir alafenamide II to recrystallization in a mixed solvent of a nitrile solvent and water or a mixed solvent of the nitrile solvent and an aromatic hydrocarbon solvent so as to obtain the high-purity tenofovir alafenamide fumarate intermediate namely tenofovir alafenamide II. The high-purity tenofovir alafenamide II has an optical purity larger than 99.50% and a chemical purity larger than 99.60%; and the crude tenofovir alafenamide fumarate intermediate namely tenofovir alafenamide II has the optical purity in a range of 60.00% to 99.00% and the chemical purity in a range of 60.00% to 99.00%. The preparation method provided by the invention has the advantages of simple and safe operation, high yield, high product purity, low production cost, and applicability to industrial production.
Reference
1 Maria Buti, Mar Riveiro-Barciela, Rafael Esteban, Tenofovir Alafenamide Fumarate: A New Tenofovir Prodrug for the Treatment of Chronic Hepatitis B Infection, The Journal of Infectious Diseases, Volume 216, Issue suppl_8, 15 October 2017, Pages S792–S796,
2 CN108440596A Novel preparation process of tenofovir alafenamide fumarate
3 CN106478725A Preparation method and application for high-purity tenofovir alafenamide fumarate intermediate
You may like
Related articles And Qustion
See also
Lastest Price from Tenofovir alafenamide hemifumarate manufacturers

US $0.00/Kg/Bag2025-04-21
- CAS:
- 1392275-56-7
- Min. Order:
- 1KG
- Purity:
- 99%min HPLC
- Supply Ability:
- 50KGS
US $0.00-0.00/kg2025-04-21
- CAS:
- 1392275-56-7
- Min. Order:
- 1kg
- Purity:
- >99% by HPLC
- Supply Ability:
- 100kg/month