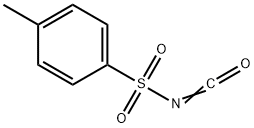
Synthesis and Application of p-Toluenesulfonyl Isocyanate
General description
p-Toluenesulfonyl isocyanate (tosyl isocyanate or -TsNCO) is a colorless liquid that reacts violently with -water and with basic and protic solvents. Reactions should be carried out with care and only in a fumehood, since TsNCO is corrosive and lachrymatory. It is easily soluble in inert media such as chlorinated, aromatic and ethereal solvents.
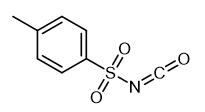
Fig. 1 The structure of p-Toluenesulfonyl Isocyanate.
Physicochemical property
p-Toluenesulfonyl isocyanate is liquid at room temperature, with a boiling point of 144°C/1.3kPa, a refractive index of 1.5340 and a flash point of 110°C. It is hygroscopic and has a tear-inducing effect.
Synthetic routes
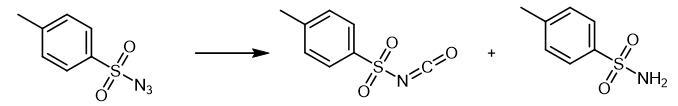
Fig. 2 The synthetic method 1 of p-Toluenesulfonyl Isocyanate.
Add the organic azide (0.75 mmol), Pd(OAc)2 (5.6 mg, 5 mol%) to an oven-dried Schlenk tube (10 mL). Purge the tube and backfill with CO (three cycles) from a balloon. Inject anhydrous MeCN (3.0 mL) into the tube. Stir at 80°C for 4 h under CO atmosphere (balloon). Concentrate the mixture under reduced pressure. Purify the residue by column chromatography (petroleum ether/EtOAc 3:1.-.1:1) [1].
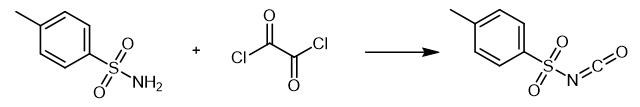
Fig. 3 The synthetic method 2 of p-Toluenesulfonyl Isocyanate.
Add oxalyl dichloride (10 cm3) dropwise to a toluene (without H2O) solution (35 cm3) of p-toluene sulfonamide (3.9381 g, 23 mmol). Add DABCO (0.26 g) as an activator to the above reaction system with stirring. Reflux the system for 6 h at 60-65 °C and reflux for 10-12 h at 90 °C. Cool to room temperature, filter the brown precipitates and evaporate the brown filtrate [2].
Application
Preparation of polymers
The chemical modification of polymers having amide moieties was carried out with p-toluenesulfonyl isocyanate. The resulting polymers revealed high hydrolytic character. For example, poly(acrylamide) was refluxed with an excess amount of p-toluenesulfonyl isocyanate in THF for 50 h to obtain a structurally modified polymer in 76% yield, whose sulfonylurea functionality was 100%. The resulting polymer was subjected to hydrolysis in a 1 M NaOH solution at 50 degrees C to convert 90% of the sulfonylurea in the side chain to the carboxylic acid moieties [3].
As Electrolyte Additive
LiNi0.5Mn1.5O4 (LNMO) is a potential cathode material for lithium-ion batteries with outstanding energy density and high voltage plateau (>4.7 V). However, the interfacial side reaction between LNMO and the liquid electrolyte seriously causes capacity fading during cycling at the high voltage. Here, p-toluenesulfonyl isocyanate (PTSI) is used as the electrolyte additive to overcome the above problem of LNMO. The results show that the specific capacity of LNMO/Li cell with 0.5 wt.% PTSI at the first cycle is effectively enhanced by 36.0 mAh/g and has better cycling performance than that without PTSI at 4.98 V. Also, a stable solid electrolyte interface (SEI) film derived from PTSI is generated on the electrode surface, which could alleviate the strike of hydrofluoric acid (HF) caused by electrolyte decomposition. These results are explained by the molecular structure of PTSI, which contains SO3. The S=O groups can delocalize the nitrogen nucleus to block the reactivity of PF5[4].
Enhance the electrospray ionization
p-Toluenesulfonyl isocyanate has been reported as a novel derivatization reagent with strong nuclephilic reactivity for the hydroxyl compounds. The derivatization for the two pharmacologically active 3-hydroxyl metabolites, 3alpha-hydroxyl-7-methyl-norethynodrel and 3beta-hydroxyl-7-methyl-norethynodrel by p-toluenesulfonyl isocyanate can be accomplished in 2 min under room temperature. The offline derivatization procedure introduced an easily ionizable sulfonylcarbamic ester moiety to the metabolites. This greatly improved the analyte's sensitivity in negative electrospray ionization and enabled us to achieve the desired lower limit of quantitation at 100 pg/ml in plasma. Therefore, a sensitive high performance liquid chromatography-mass spectrometry (HPLC-MS) method for the analysis of the two stereo isomers was developed. The method had been validated to be accurate, precise, and sensitive, and can be used for the metabolism pharmacokinetic study of tibolone in human subjects [5].
References
[1] Gu Z Y, Wu Y, Jin F, et al. Intermolecular C–H Amidation of Alkenes with Carbon Monoxide and Azides via Tandem Palladium Catalysis[J]. Synthesis, 2021, 53(18): 3361-3371.
[2] Xi P, Liu X, Lu H, et al. Synthesis, characterization and DNA-binding studies of 1-(pyridin-2-yl)-3-tosylurea and its NdIII, EuIII complexes[J]. Transition Metal Chemistry, 2007, 32(6): 757-761.
[3] Iwamura T, Tomita I, Suzuki M, et al. Reaction of p‐toluenesulfonyl isocyanate with polymers having amide moieties and hydrolysis of the obtained polymers[J]. Journal of Polymer Science Part A: Polymer Chemistry, 2000, 38(18): 3440-3449.
[4] Wang R H, Li X H, Wang Z X, et al. Electrochemical analysis for enhancing interface layer of spinel Li4Ti5O12: p-toluenesulfonyl isocyanate as electrolyte additive[J]. ACS Applied Materials & Interfaces, 2015, 7(42): 23605-23614.
[5] Zuo M, Gao M, Liu Z, et al. p-Toluenesulfonyl isocyanate as a novel derivatization reagent to enhance the electrospray ionization and its application in the determination of two stereo isomers of 3-hydroxyl-7-methyl-norethynodrel in plasma[J]. Journal of Chromatography B, 2005, 814(2): 331-337.
Related articles And Qustion
See also
Lastest Price from p-Toluenesulfonyl Isocyanate manufacturers
US $0.00/kg2025-05-15
- CAS:
- 4083-64-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS

US $100.00-75.00/kg2025-04-21
- CAS:
- 4083-64-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000