The preparation of riboflavin sodium phosphate
Introduction
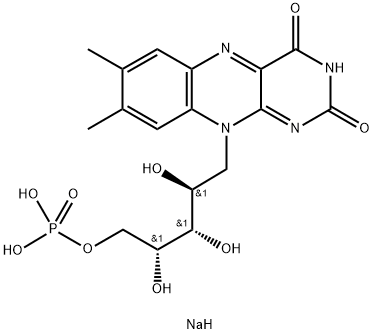
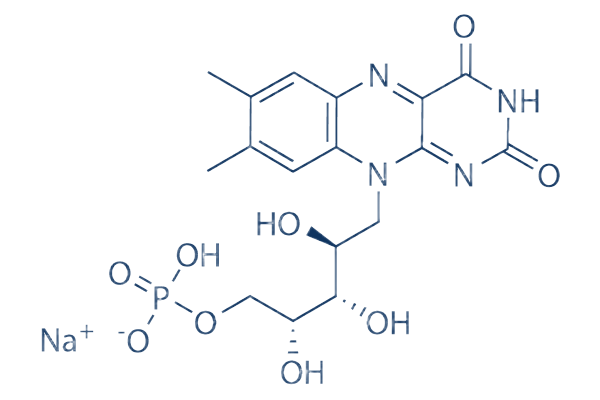
Riboflavin sodium phosphate is a ribitol phosphate. It derives from a riboflavin. Riboflavin 5'-Phosphate Sodium is the phosphate sodium salt form of riboflavin, a water -soluble and essential micronutrient that is the principal growth-promoting factor in naturally occurring vitamin B complexes. Riboflavin phosphate sodium is a water-soluble, essential micronutrient that is the principal growth-promoting factor in naturally occurring vitamin B complexes. Riboflavin sodium phosphate is a supplement form of riboflavin (vitamin B2), which is necessary for human nutrition, and growth. It finds potential applications in biological redox reactions and is found in several foodstuffs, energy drinks, and vitamin formulations. Riboflavin sodium phosphate may be used as an analytical reference standard for the determination of the analyte in pharmaceutical formulations by liquid chromatography. This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.

Picture 1 Riboflavin sodium phosphate powders
Riboflavin sodium phosphate compound injection and preparing method thereof
The invention aims to provide a riboflavin sodium phosphate compound, a riboflavin sodium phosphate compound injection and a preparation method thereof[1]. The riboflavin sodium phosphate having a novel crystal structure is better in stability, is reduced in phosphorylation reaction time during a synthetic process and is increased in yield. The riboflavin sodium phosphate compound is suitable for preparing a medicinal composition since the riboflavin sodium phosphate compound is uniform in particle size, is excellent in stability and is high in yield. The riboflavin sodium phosphate compound can be prepared into various known dosage forms, such as a freeze-dried powder needle, an injection and the like.
Method and process for synthesizing riboflavin sodium phosphate
The invention relates to a method for producing a medical product, in particular to a process for synthesizing riboflavin sodium phosphate and belongs to the technical field of medicinal chemistry[2]. The process mainly comprises the following steps that riboflavin and phosphorus oxychloride are subjected to an esterification reaction under certain conditions to generate riboflavin phosphate; the riboflavin phosphate is filtered to remove solid materials; the filtrate is hydrolyzed to generate the crude product of riboflavin sodium phosphate; the crude product of riboflavin sodium phosphate is filtered; the filter cakes are collected and crystallized to generate riboflavin sodium phosphate crystals; the riboflavin sodium phosphate crystals are filtered; the filter cakes are dried in a drier to obtain the product. The method is available in raw materials, low in cost, simple in reaction, easy to control and high in yield, and can be applied to batch production in workshops. The method has a wide application prospect in the aspect of synthesizing process of the riboflavin sodium phosphate.
Clinical Investigation in Effect of Riboflavin Sodium Phosphate To investigate the clinical effect of riboflavin sodium phosphate on prevention of radiotherapy related esophagitis (RRE)[3]. Methods: This retrospective study involved 55 patients with middle and advanced esophageal cancer who were divided into an experimental group of 28 and a control group of 27 patients. Those in the experimental group were treated with riboflavin sodium phosphate combined with conventional symptomatic treatment during radiotherapy; while patients in control group received the latter alone. The incidence and degree of RRE were compared after radiotherapy. Results: The incidences of RRE in experimental and control group were 53.5% and 81.4%, respectively (p<0.05); the incidence of stages III and IV RRE in the experimental group was 17.8%, while in the control group it was 44.4% (p<0.05). Conclusion: Riboflavin sodium phosphate could significantly prevent RRE and reduce the incidence of stage III and IV disease. These results were worthy of further confirmation by randomized controlled trials.
Reference
1 CN104327120A Riboflavin sodium phosphate compound injection and preparing method thereof
2 CN105273008A Method and process for synthesizing riboflavin sodium phosphate
3 Shen K, Huang X E. Clinical investigation in effect of riboflavin sodium phosphate on prevention and treatment for patients with radiotherapy related esophagitis[J]. Asian Pacific Journal of Cancer Prevention, 2015, 16(4): 1525-1527.
Related articles And Qustion
See also
Lastest Price from Riboflavin 5'-Monophosphate Sodium Salt manufacturers

US $0.00/KG2025-08-19
- CAS:
- 130-40-5
- Min. Order:
- 25KG
- Purity:
- 99%HPLC,USP Standard
- Supply Ability:
- 200ton

US $0.00-0.00/kg2025-04-23
- CAS:
- 130-40-5
- Min. Order:
- 1kg
- Purity:
- 73.0%~79.0%; USP34
- Supply Ability:
- 5000kg/month