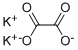
The anhydrous salt, mol wt 166.22, is obtained when the monohydrate is dehydrated at 160 °C. The monohydrate is preferred as a reagent in analytical chemistry and in miscellaneous uses principally because of its high solubility as compared with other simple neutral oxalates.
Colorless, transparent crystals; odorless.
Soluble in water.
Cleaning and bleaching straw, removing stains in photography; in vitro blood anticoagulant; also in analytical chemistry.
Potassium oxalate is a white crystal or powder made by
neutralizing oxalic acid with potassium carbonate. It is soluble
in water 1:3 but not in alcohol. Potassium oxalate was used as
an early developer for gelatin plates but is best known as the
developer for platinum prints.
Potassium oxalate, K2C204, H20, is odorless, efforescent, water-soluble, colorless crystals that decompose when heated. Sinks in and mixes slowly with water. Used in analytical chemistry and photography, and as a bleach and oxalic acid source.
Potassium oxalate gives basic aqueous solutions. Reacts as a base to neutralize acids in reactions that generate heat, but less than is generated by neutralization of the bases in reactivity group 10. Can serve as a reducing agent in reactions that generate carbon dioxide.
Toxic by inhalation and ingestion.
Inhalation of dust can cause systemic poisoning. Ingestion causes burning pain in throat, esophagus, and stomach; exposed areas of mucous membrane turn white; vomiting, severe purging, weak pulse, and cardiovascular collapse may result; if death is delayed, neuromuscular symptoms develop. Contact with eyes or skin causes irritation.
Behavior in Fire: Loses water at about 160° and decomposes to carbonate with no charring. The reaction is not hazardous.
Flammability and Explosibility
Not classified