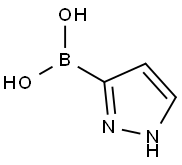
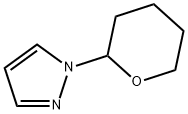
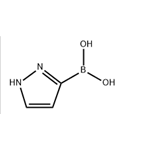
General procedure for the synthesis of 1H-pyrazole-3-boronic acid from 1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazole: To a solution of 1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazole (7.6 g, 52 mmol) in tetrahydrofuran (THF, 50 mL) was added slowly at -78 °C n-butyllithium (n-BuLi, 33 mL, 2.5 M hexane solution, 82.5 mmol), followed by the addition of triisopropylborane (12.7 mL, 55 mmol) dropwise, keeping the reaction temperature at -70 °C. The reaction mixture was stirred at -70 °C for 1 hour and then slowly warmed to room temperature over 4 hours. After completion of the reaction, the reaction was quenched with 2M hydrochloric acid (HCl) and the solvent was removed under vacuum. The pH of the reaction mixture was adjusted to 6 with 1M sodium hydroxide (NaOH) solution, at which time a precipitate was formed. The precipitate was collected by filtration, washed sequentially with toluene and petroleum ether, and finally ground with ethyl acetate to afford 1H-pyrazole-3-boronic acid as a white solid (2.7 g, 48% yield), and the product could be used in subsequent reactions without further purification.