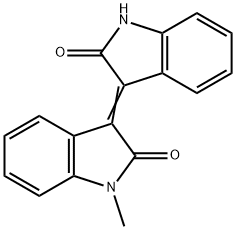
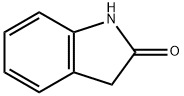
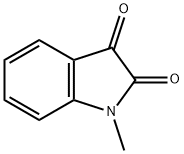
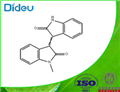
GENERAL METHOD: A mixture of 1-methylisatin (1, 1.0 mmol), 2-indolone (2, 1.0 mmol) and zirconium tetrachloride (ZrCl4, 23 mg, 0.1 mmol) in anhydrous ethanol (5 mL) was heated under reflux conditions. The progress of the reaction was monitored by thin layer chromatography (TLC) and after complete disappearance of the reactants, which usually takes 8-12 h, the reaction mixture was slowly cooled to room temperature. The precipitated red solid was collected by filtration and washed with a small amount of anhydrous ethanol to give pure 1-methyl-[3,3'-bi-indolinylidene]-2,2'-dione (3).