Sitagliptin phosphate is dipeptidyl peptidase Ⅳ(DDP-4) inhibitor class of drugs developed by the German Merck company and firstly obtained the US Food and Drug Administration approval for the treatment of type 2 diabetes, is a new antidiabetic drug, can improve the body's own ability to reduce high blood glucose levels, and the relatively increase naturally occurring incretin by inhibiting the activity of this enzyme, including the levels of glucagon-like peptide 1 and glucose-dependent insulinotropic peptide, thereby triggering the pancreas to improve insulin production and stop glucose production in liver, and ultimately reduce the clinical effect of blood glucose concentration. Features of this product is to stimulate insulin secretion, at the same time can alleviate hunger, but does not make weight gain, and also cannot happen hypoglycemia and edema, is not suitable for use in diabetic patients with poor glycemic control and often developed hypoglycemia. By verification of clinical 552 cases of mild to moderate type 2 diabetes, taken once a day Sitagliptin phosphate, once time 100 mg, after taking the drug for 12 weeks, can make glycated hemoglobin reduce 0.6%-1.1%. Incidence of adverse reactions is similar to placebo, the most frequently reported adverse reactions (incidence> 5% and greater than placebo) is a stuffy or runny nose, and sore throat, upper respiratory tract infection and headache.
Clinical studies showed that the Sitagliptin phosphate as monotherapy in patients with type 2 diabetes, can make glycated hemoglobin (HbA1c) levels significantly reduce. When in combination with the metformin or TZDs, has a significant role of adjuvant therapy, can focus on three kinds of major defect in type 2 diabetes: insulin resistance, β-cell dysfunction (reduction of insulin release), and α-cell dysfunction (unsuppressed hepatic glucose generation) plays a role. But the drug should not be used for treatment of patients with type 1 diabetes or diabetic ketoacidosis.
The above information is edited by the chemicalbook of Liu Yujie.
Sitagliptin phosphate (trade name Januvia) is the DPP-4 inhibitor first clinically approved for the treatment of type 2 diabetes, developed by Germany company Merck, and was listed in 2006 in Mexico and the United States in 2007, also obtained EU approval for the treatment of type 2 diabetes. China approval was soon, currently Sitagliptin phosphate tablets has become the second largest drug of oral diabetes drugs in US.
Sitagliptin phosphate can enhance a called incretin system of human physiological system, resulting in physiological hypoglycemic effect. This physiological system itself can affect β cells and α cells of the islet, help to regulate glucose, and insulin reduction only caused by β-cell dysfunction can cause the increase of blood glucose, or because cells α and β-cell dysfunction cause loss of control in hepatic glucose synthesis and thus lead to elevated blood glucose, DPP-4 will produce efficacy.
Sitagliptin phosphate exists dependent of glucose levels, it will not only know hypoglycemic blindly, not predatorily press islet, exhaust the pancreas function. In contrast, the test proved that it has the prospect of long-term protection of human β cells. In addition, it does not lead to side effects such as weight gain, decreased blood glucose, medication compliance of patient will be greatly enhanced. Therefore, in theory it's effective role in life will greatly exceed the current oral antidiabetic drug, insulin dependent of patients will be greatly delayed.
Sitagliptin is the first novel dipeptidyl peptidase IV inhibitor
from Merck for the treatment of type 2 diabetes without
weight gain and the incidence of hypoglycemia was similar
to placebo. Sitagliptin acts by enhancing the body’s incretin
system, which helps to regulate glucose by affecting β
and α cells in the pancreas.
Sitagliptin is a trizolopyrazine dipeptidyl peptidase IV inhibitor. Sitagliptin has recently been approved for the therapy of type II diabetes.
A trizolopyrazine dipeptidyl peptidase IV inhibitor. It has recently been approved for the therapy of type II diabetes.
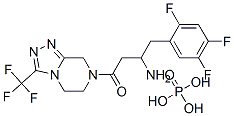
Sitagliptin phosphate is the 1:1 phosphoric acid salt ofsitagliptin free base (i.e., (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-1-(2,4,5-trifluorophenyl)-2-butanamine), and is marketed(Junuvia, 2006) in 25-, 50-, and 100-mg tablets. Acombination product with metformin (Janumet, 2007) intwo strengths (50 mg/500 mg and 50 mg/1,000 mgsitagliptin/metformin) is also available, and sitagliptin mayalso be prescribed with a thiazolidinedione, or possibly a sulfonylurea.The phosphate salt provides very high water solubility.The bioavailability of orally administered sitagliptinis~87%. The drug exhibits relatively low plasma protein binding (~38%), a relatively large volume of distribution(198 L), and a terminal elimination half-life of 12 hours.About 79% of a 100-mg oral dose is excreted unchanged inurine, the balance as trace-level metabolites (CYP3A4, lessercontribution by CYP2C8) in urine or feces: 87% of administeredradioactivity is excreted in urine, and 13% in feces.Active tubular excretion is reported to play a key role in renalclearance of unchanged drug, and may be mediated at least inpart via the organic anion transporter hOAT-3.
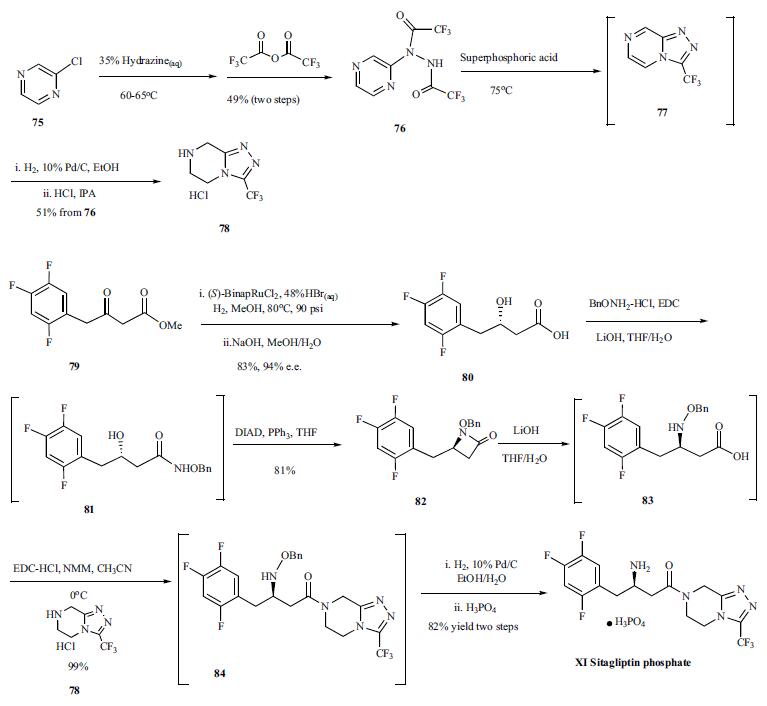
Synthesis of sitagliptin
started with the slow addition of chloropyrazine (75)
to 35% aqueous hydrazine at 60-65C, controlling this exothermic
reaction and making it process-friendly, and the resulting
crude pyrazinyl hydrazine was acetylated with trifluoroacetic
anhydride to afford bis-trifluoromethylhydrazide
76 in 49% yield from the chloropyrazine. Compound
76 was treated with superphosphoric acid, a diluted
form of polyphosphoric acid, to give cyclized compound 77
which was hydrogenated with Pd/C and the resulting product
was treated with HCl in IPA to afford compound 78 as its
HCl salt in 51% yield from 76. Compound 78 was used later
on in a coupling reaction to generate sitagliptin. Compound
79, a beta-ketoester, was subjected to asymmetric reduction
with (S)-BinapRuCl2-triethylamine complex in methanol at
80C, catalytic amount of hydrogen bromide, and 90 psi of
hydrogen atmosphere to give the desired beta-hydroxy ester which was hydrolyzed to give carboxylic acid 80 in 94% e.e.
and 83% yield. The carboxylic acid 80 was coupled with
BnONH2-HCl in the presence of EDC and lithium hydroxide
in THF/H2O to give coupled compound 81 which was cyclized
to compound 82 with DIAD and triphenylphosphine
in THF in 81% yield from compound 80. Compound 82 was
then hydrolyzed to |-amino acid 83 with lithium hydroxide,
and the acid was coupled with compound 78 at 0C with
EDC-HCl and NMM as base to give compound 84 in excellent
yield. Compound 84 was hydrogenated with 10% Pd/C
in an ethanol/H2O mix solvent system. The water was crucial
to complete the reaction and restore catalyst activity. Finally,
the ethanol solution of the hydrogenated product was treated
with phosphoric acid, and sitagliptin (XI) was crystallized as
its anhydrous phosphoric acid salt from aqueous ethanol solution.
sitagliptin was a potent inhibitor for dpp-4 with an ic50 of 18 nm [1]. sitagliptin inhibited dpp-8 with an ic50 of 48 μm. sitagliptin showed no effect on several related peptidases, including dpp-9, dpp-ii, and amino peptidase p [1].
[1] biftu t, feng d, qian x, et al. (3r)-4-[(3r)-3-amino-4-(2, 4, 5-trifluorophenyl) butanoyl]-3-(2, 2, 2-trifluoroethyl)-1, 4-diazepan-2-one, a selective dipeptidyl peptidase iv inhibitor for the treatment of type 2 diabetes[j]. bioorganic & medicinal chemistry letters, 2007, 17(1): 49-52.
[2] fleischer b. cd26: a surface protease involved in t-cell activation[j]. immunology today, 1994, 15(4): 180-184.
[3] aschner p, kipnes m s, lunceford j k, et al. effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes[j]. diabetes care, 2006, 29(12): 2632-2637.
[4] green j b, bethel m a, armstrong p w, et al. effect of sitagliptin on cardiovascular outcomes in type 2 diabetes[j]. new england journal of medicine, 2015, 373(3): 232-242.