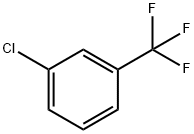
3-Chlorobenzotrifluoride
- Product Name3-Chlorobenzotrifluoride
- CAS98-15-7
- MFC7H4ClF3
- MW180.55
- EINECS202-642-9
- MOL File98-15-7.mol
Chemical Properties
| Melting point | -56 °C (lit.) |
| Boiling point | 137-138 °C (lit.) |
| Density | 1.331 g/mL at 25 °C |
| refractive index | n |
| Flash point | 97 °F |
| form | clear liquid |
| color | Colorless to Almost colorless |
| Specific Gravity | 1.336 |
| Water Solubility | <0.1 g/100 mL at 22 ºC |
| BRN | 510215 |
| Exposure limits | ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3 |
| InChI | InChI=1S/C7H4ClF3/c8-6-3-1-2-5(4-6)7(9,10)11/h1-4H |
| InChIKey | YTCGOUNVIAWCMG-UHFFFAOYSA-N |
| SMILES | C1(Cl)=CC=CC(C(F)(F)F)=C1 |
| CAS DataBase Reference | 98-15-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzene, 1-chloro-3-(trifluoromethyl)-(98-15-7) |
| EPA Substance Registry System | 3-Chlorobenzotrifluoride (98-15-7) |
Safety Information
| Hazard Codes | Xi,F |
| Risk Statements | 10-36/37/38 |
| Safety Statements | 16-26-36-24/25 |
| RIDADR | UN 2234 3/PG 3 |
| WGK Germany | 1 |
| RTECS | XS9142000 |
| Hazard Note | Flammable/Irritant |
| TSCA | TSCA listed |
| HazardClass | 3 |
| PackingGroup | III |
| HS Code | 29039990 |
| Storage Class | 3 - Flammable liquids |
| Hazard Classifications | Aquatic Chronic 3 Flam. Liq. 3 |

