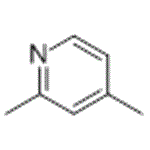
2,4-Lutidine
- Product Name2,4-Lutidine
- CAS108-47-4
- MFC7H9N
- MW107.15
- EINECS203-586-8
- MOL File108-47-4.mol
Chemical Properties
| Melting point | -60 °C (lit.) |
| Boiling point | 159 °C (lit.) |
| Density | 0.927 g/mL at 25 °C (lit.) |
| vapor pressure | 3.28hPa at 25℃ |
| FEMA | 4389 | 2,4-DIMETHYLPYRIDINE |
| refractive index | n |
| Flash point | 99 °F |
| storage temp. | Flammables area |
| solubility | 350g/l |
| pka | 6.99(at 25℃) |
| form | Liquid |
| color | Colorless to Light yellow to Light orange |
| Odor | at 0.01 % in dipropylene glycol. smoky phenolic |
| Odor Type | smoky |
| Water Solubility | 15 g/100 mL (20 ºC) |
| Sensitive | Hygroscopic |
| JECFA Number | 2151 |
| BRN | 1506 |
Safety Information
| Hazard Codes | T,F,Xi |
| Risk Statements | 10-23/24/25-36/37/38-25-20/21-20/21/22 |
| Safety Statements | 16-26-36/37/39-45 |
| RIDADR | UN 1992 3/PG 3 |
| WGK Germany | 3 |
| RTECS | OK9400000 |
| F | 8 |
| TSCA | TSCA listed |
| HazardClass | 3 |
| PackingGroup | III |
| HS Code | 29333999 |
| Storage Class | 3 - Flammable liquids |
| Hazard Classifications | Acute Tox. 3 Dermal Acute Tox. 3 Inhalation Acute Tox. 3 Oral Eye Irrit. 2 Flam. Liq. 3 Skin Irrit. 2 STOT SE 3 |