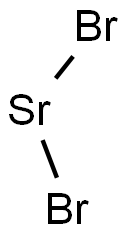
Strontium bromide
- Product NameStrontium bromide
- CAS10476-81-0
- MFBr2Sr
- MW247.43
- EINECS233-969-5
- MOL File10476-81-0.mol
Chemical Properties
| Melting point | 643°C |
| Density | 4,216 g/cm3 |
| form | powder |
| color | White |
| Specific Gravity | 4.216 |
| Water Solubility | Soluble in water, carbinol, ethanol, amyl alcohol. Insoluble in ether. |
| Sensitive | Hygroscopic |
| Merck | 14,8837 |
| Stability | hygroscopic |
| Major Application | electroplating material synthesis precursor |
| InChI | 1S/2BrH.Sr/h2*1H;/q;;+2/p-2 |
| InChIKey | YJPVTCSBVRMESK-UHFFFAOYSA-L |
| SMILES | Br[Sr]Br |
| CAS DataBase Reference | 10476-81-0(CAS DataBase Reference) |
| EPA Substance Registry System | Strontium bromide (SrBr2) (10476-81-0) |
Safety Information
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36 |
| WGK Germany | 3 |
| RTECS | WK8050000 |
| TSCA | TSCA listed |
| HS Code | 2827590000 |
| Storage Class | 11 - Combustible Solids |
| Hazard Classifications | Eye Irrit. 2 Skin Irrit. 2 STOT SE 3 |
