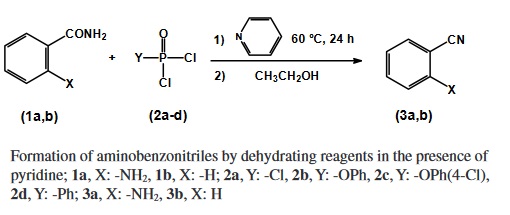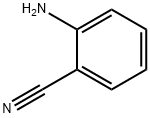
2-Aminobenzonitrile
- Product Name2-Aminobenzonitrile
- CAS1885-29-6
- MFC7H6N2
- MW118.14
- EINECS217-549-9
- MOL File1885-29-6.mol
Chemical Properties
| Melting point | 45-48 °C (lit.) |
| Boiling point | 267-268 °C (lit.) |
| Density | 1.11 g/cm3 (50℃) |
| refractive index | 1.5500 (estimate) |
| Flash point | >230 °F |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) |
| form | Crystalline Flakes |
| pka | 0.77(at 25℃) |
| color | Yellow to beige-brown |
| Water Solubility | INSOLUBLE |
| BRN | 907187 |
| InChI | 1S/C7H6N2/c8-5-6-3-1-2-4-7(6)9/h1-4H,9H2 |
| InChIKey | HLCPWBZNUKCSBN-UHFFFAOYSA-N |
| SMILES | Nc1ccccc1C#N |
| LogP | 0.901 |
| CAS DataBase Reference | 1885-29-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzonitrile, 2-amino-(1885-29-6) |
Safety Information
| Hazard Codes | Xn,T,Xi |
| Risk Statements | 20/21/22-36/37/38-43 |
| Safety Statements | 26-36/37-36 |
| RIDADR | 3276 |
| WGK Germany | 2 |
| RTECS | CB4575000 |
| TSCA | TSCA listed |
| HazardClass | 6.1 |
| PackingGroup | III |
| HS Code | 29269095 |
| Storage Class | 13 - Non Combustible Solids |
| Hazard Classifications | Acute Tox. 4 Dermal Acute Tox. 4 Inhalation Acute Tox. 4 Oral Eye Irrit. 2 Skin Irrit. 2 Skin Sens. 1 STOT SE 3 |
| Toxicity | LD50 ivn-mus: 180 mg/kg CSLNX* NX#00381 |