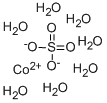
Cobalt sulfate heptahydrate
- Product NameCobalt sulfate heptahydrate
- CAS10026-24-1
- MFCoH14O11S
- MW281.1
- EINECS600-050-9
- MOL File10026-24-1.mol
Chemical Properties
| Melting point | 98 °C |
| Boiling point | 735°C |
| Density | 2.03 g/mL at 25 °C(lit.) |
| bulk density | 900kg/m3 |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: soluble |
| form | Solid |
| color | Red-brown |
| Specific Gravity | 1.948 |
| PH | 4 (100g/l, H2O, 20℃) |
| Water Solubility | 362 g/L (20 ºC) |
| Merck | 14,2448 |
| Exposure limits | ACGIH: TWA 0.02 mg/m3 |
| Stability | Stable. Non-flammable. Hygroscopic. Dehydrates at around 41 C and 71 C. |
| InChI | 1S/Co.H2O4S.H2O/c;1-5(2,3)4;/h;(H2,1,2,3,4);1H2/q+2;;/p-2 |
| InChIKey | MEYVLGVRTYSQHI-UHFFFAOYSA-L |
| SMILES | O.[Co++].[O-]S([O-])(=O)=O |
| LogP | -1.031 (est) |
Safety Information
| Hazard Codes | T,N |
| Risk Statements | 49-42/43-51/53-50/53-22-68-60 |
| Safety Statements | 53-23-36/37-45-61-60-22 |
| RIDADR | UN 3082 9/PG 3 |
| WGK Germany | 2 |
| RTECS | GG3200000 |
| TSCA | Yes |
| HazardClass | 9 |
| PackingGroup | III |
| HS Code | 28332930 |
| Storage Class | 6.1D - Non-combustible acute toxic Cat.3 toxic hazardous materials or hazardous materials causing chronic effects |
| Hazard Classifications | Acute Tox. 4 Oral Aquatic Acute 1 Aquatic Chronic 1 Carc. 1B Inhalation Eye Irrit. 2 Muta. 2 Repr. 1B Resp. Sens. 1 Skin Sens. 1 |
| Toxicity | LD50 orally in Rabbit: 582 mg/kg |


