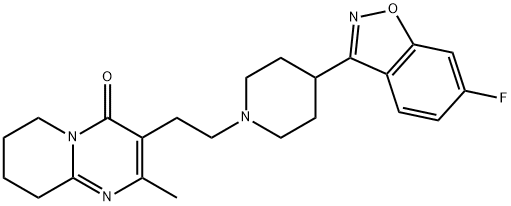
Risperdal
- Product NameRisperdal
- CAS106266-06-2
- MFC23H27FN4O2
- MW410.48
- EINECS600-733-1
- MOL File106266-06-2.mol
Chemical Properties
| Melting point | 170°C |
| Boiling point | 572.4±60.0 °C(Predicted) |
| Density | 1.38±0.1 g/cm3(Predicted) |
| Flash point | 9℃ |
| storage temp. | 2-8°C |
| solubility | DMSO: ≥5mg/mL |
| pka | pKa 8.3 (Uncertain) |
| form | powder |
| color | white to off-white |
| Water Solubility | 44.74mg/L(25 ºC) |
| Merck | 14,8233 |
| BCS Class | 2 |
| CAS DataBase Reference | 106266-06-2(CAS DataBase Reference) |
Safety Information
| Hazard Codes | T,F |
| Risk Statements | 25-39/23/24/25-23/24/25-11 |
| Safety Statements | 28-36-45-36/37-16 |
| RIDADR | 3249 |
| WGK Germany | 3 |
| RTECS | UV1164800 |
| HazardClass | 6.1(a) |
| PackingGroup | II |
| HS Code | 29333990 |
| Hazardous Substances Data | 106266-06-2(Hazardous Substances Data) |
| Toxicity | LD50 in male, female mice, rats, dogs (mg/kg): 29.7, 26.9, 34.3, 35.4, 14.1, 18.3 i.v.; 82.1, 63.1, 113, 56.6, 18.3, 18.3 orally (Janssen, 1988) |
Usage And Synthesis
Characters: 1mg Risperidone tablets are white film-coated tablets, after removing the coating ,they are white; 2mg Risperidone tablets are pale orange film-coated tablets,after removing the coating , they are white.
Pharmacological characteristics: This product is benzisoxazole derivative,it is a new generation of antipsychotics. Its active ingredient Risperidone is a selective monoamine antagonist with unique properties, it has a high affinity with the serotonin 5-HT2 receptors and the dopamine D2 receptors . Risperidone can also be combined with α1-adrenergic receptors , and it can combine with the histamine H1-receptors and α2-adrenoceptors with lower affinity . Risperidone can not combine with cholinergic receptors. Risperidone is a potent D2 antagonist, which can improve the positive symptoms of schizophrenia, but inhibition of motor function, as well as catalepsy caused by it are lesser than classical antipsychotics. Balance of serotonin and dopamine antagonism of the central nervous systemcan can reduce the potential for extrapyramidal side effects, and expand its role in the treatment of negative symptoms and affective symptoms of schizophrenia.
Pharmacokinetic characteristics: after oral administration,Risperidone can be completely absorbed and the peak of plasma concentration is reached within 1-2 hours, and its absorption is not affected by food. In vivo, Risperidone is metabolized to 9-hydroxy portion-Risperidone, which has similar pharmacological effects to Risperidone. This product can be quickly distributed in the body, Risperidone plasma protein binding rate is 88%, 9-hydroxy-Risperidone plasma protein binding rate is 77%. The drug elimination half-life is about three hours, antipsychotic active ingredient elimination half-life is 24 hours. Most patients reach a steady state of Risperidone in a day, and after 4-5 days ,they reach the 9-hydroxy-risperidone homeostasis. One week after treatment, 70% of the drug is excreted in urine and 14% of the drug is through fecal excretion, 35-45% of the section of urinary excretion are Risperidone and 9-hydroxy-risperidone, the remaining is non-active metabolite . Elderly patients and patients with renal insufficiency have higher plasma concentrations of Risperidone, so they have slower clearance.
Pharmacological characteristics: This product is benzisoxazole derivative,it is a new generation of antipsychotics. Its active ingredient Risperidone is a selective monoamine antagonist with unique properties, it has a high affinity with the serotonin 5-HT2 receptors and the dopamine D2 receptors . Risperidone can also be combined with α1-adrenergic receptors , and it can combine with the histamine H1-receptors and α2-adrenoceptors with lower affinity . Risperidone can not combine with cholinergic receptors. Risperidone is a potent D2 antagonist, which can improve the positive symptoms of schizophrenia, but inhibition of motor function, as well as catalepsy caused by it are lesser than classical antipsychotics. Balance of serotonin and dopamine antagonism of the central nervous systemcan can reduce the potential for extrapyramidal side effects, and expand its role in the treatment of negative symptoms and affective symptoms of schizophrenia.
Pharmacokinetic characteristics: after oral administration,Risperidone can be completely absorbed and the peak of plasma concentration is reached within 1-2 hours, and its absorption is not affected by food. In vivo, Risperidone is metabolized to 9-hydroxy portion-Risperidone, which has similar pharmacological effects to Risperidone. This product can be quickly distributed in the body, Risperidone plasma protein binding rate is 88%, 9-hydroxy-Risperidone plasma protein binding rate is 77%. The drug elimination half-life is about three hours, antipsychotic active ingredient elimination half-life is 24 hours. Most patients reach a steady state of Risperidone in a day, and after 4-5 days ,they reach the 9-hydroxy-risperidone homeostasis. One week after treatment, 70% of the drug is excreted in urine and 14% of the drug is through fecal excretion, 35-45% of the section of urinary excretion are Risperidone and 9-hydroxy-risperidone, the remaining is non-active metabolite . Elderly patients and patients with renal insufficiency have higher plasma concentrations of Risperidone, so they have slower clearance.
It is obtained from dimethylformamide-isopropyl crystallization, m.p. 170.0 ℃. Acute toxicity LD50 male and female mice, male and female rats, male and female dogs (mg/kg): 29.7,26.9,34.3,35.4,14.1,18.3 intravenous injection; 82.1,63.1,113,56.6,18.3,18.3 oral.
1. It has antagonism effect on 5HT2 receptors and D2 receptors. It is used for acute and chronic schizophrenia.
2. Antipsychotic drugs.
2. Antipsychotic drugs.
Risperidone advantages:
(1)it is effective not only for positive symptoms, but also effective for negative symptoms, it can also improve cognitive disorders, thereby improving the patient's condition as a whole.
(2) adverse reactions of the drug is light,it produces fewer extrapyramidal side effects.
(3)it is easy to adjust the dose, for most patients, the dose is 2~6mg/d, which can produce a therapeutic effect.
(4) for some poor therapeutic effect of conventional antipsychotics or intolerant patients, Risperidone may produce better results.
(5)Compliance of patients greatly increases during the period of medication .
(6) foreign research shows, Risperidone can reduce the expenses of patients and reduce the overall expenditure of the patients, and thus more in line with the principles of health economics.
Risperidone side effects:
Risperidone common adverse reactions: insomnia, anxiety, agitation, headache; rare adverse reactions are: drowsiness, fatigue, dizziness, decreased attention, constipation, indigestion, nausea, vomiting, abdominal pain, blurred vision, priapism, erectile dysfunction, weak ejaculation, sexual apathy, urinary incontinence, rhinitis, rash and other allergic reactions. Risperidone can cause extrapyramidal side effects, tremors, rigidity, salivation, bradykinesia, akathisia, acute dystonia, orthostatic hypotensionlactation,lactation, gynecomastia, menstrual disorders, amenorrhea can occur occasionally, Risperidone can also cause weight gain, edema, and increased liver enzymes and so on.
(1)it is effective not only for positive symptoms, but also effective for negative symptoms, it can also improve cognitive disorders, thereby improving the patient's condition as a whole.
(2) adverse reactions of the drug is light,it produces fewer extrapyramidal side effects.
(3)it is easy to adjust the dose, for most patients, the dose is 2~6mg/d, which can produce a therapeutic effect.
(4) for some poor therapeutic effect of conventional antipsychotics or intolerant patients, Risperidone may produce better results.
(5)Compliance of patients greatly increases during the period of medication .
(6) foreign research shows, Risperidone can reduce the expenses of patients and reduce the overall expenditure of the patients, and thus more in line with the principles of health economics.
Risperidone side effects:
Risperidone common adverse reactions: insomnia, anxiety, agitation, headache; rare adverse reactions are: drowsiness, fatigue, dizziness, decreased attention, constipation, indigestion, nausea, vomiting, abdominal pain, blurred vision, priapism, erectile dysfunction, weak ejaculation, sexual apathy, urinary incontinence, rhinitis, rash and other allergic reactions. Risperidone can cause extrapyramidal side effects, tremors, rigidity, salivation, bradykinesia, akathisia, acute dystonia, orthostatic hypotensionlactation,lactation, gynecomastia, menstrual disorders, amenorrhea can occur occasionally, Risperidone can also cause weight gain, edema, and increased liver enzymes and so on.
It is used for the treatment of acute and chronic schizophrenia and other various psychotic state obvious positive symptoms (such as hallucinations, illusions, thought disorder, hostility, suspicion) and significant negative symptoms (such as unresponsiveness, emotional apathy and social apathy, of few words). Also it can alleviate affective symptoms associated with schizophrenia (such as: depression, guilt, anxiety). For patients whom Risperidone is effective to for acute treatment ,in the maintenance phase of treatment, the Risperidone can continue to play its clinical efficacy.
1. It can antagonize levodopa and other dopamine agonists role.
2. carbamazepine and other hepatic enzyme inducers may reduce the plasma concentration of the active ingredient of the product, once stop using carbamazepine or other hepatic enzyme inducers, the product should be re-determined its dose ,reduction if necessary .
3. phenothiazines, tricyclic antidepressants, and some β-blockers may increase the plasma concentration of this product, but they do not increase the plasma concentration of the antipsychotic active ingredient.
4. When taken together with other highly protein bound drugs , there is no clinically significant mutual replacement of plasma protein.
2. carbamazepine and other hepatic enzyme inducers may reduce the plasma concentration of the active ingredient of the product, once stop using carbamazepine or other hepatic enzyme inducers, the product should be re-determined its dose ,reduction if necessary .
3. phenothiazines, tricyclic antidepressants, and some β-blockers may increase the plasma concentration of this product, but they do not increase the plasma concentration of the antipsychotic active ingredient.
4. When taken together with other highly protein bound drugs , there is no clinically significant mutual replacement of plasma protein.
1-Acetyl-4-methyl-piperidine chloride and difluorobenzene react under aluminum trichloride catalysis, after acylation, use hydrochloric acid hydrolysis to deacetylate in piperidine ring, and then after hydroxylamine ,use base to catalyze, after cyclization , get benzisoxazole derivatives. 4.4 parts of the isoxazole derivatives, 5.3 parts of ethyl 3-chloro-2-methyl-4H-pyrido [1,2-α] pyrimidin-4-one hydrochloride, 8 parts of sodium carbonate and 0.1 parts of potassium iodide, in dimethyl formamide , heat at 85-90 ℃, Risperidone is generated, 46% yield.
For treatment of newly diagnosed or recurrent acute schizophrenia, new antipsychotic efficacy are good, but the current maintenance therapy for acute treatment duration and dose has not yet been unified global consensus. This study is designed to investigate, ,the effects that the use of an effective dose of Risperidone maintenance treatment for different time have on disease recurrence after the acute phase treatment of schizophrenia patients is completed , and verify the safety and tolerability long-term treatment with Risperidone.
The multicenter open randomized controlled study is participated by the National 19 psychiatric hospitals or psychiatric general hospitals, Objects of study are patients in line with the American Diagnostic and Statistical Manual Mental Disorders 4th edition (DSM-Ⅳ) schizophrenia diagnostic criteria , with previous episodes at least once,the total treatment time from this episode to join to the group, is ≤5 months,and they have been completed acute treatment and the symptoms are under control, they have been using fixed-dose Risperidone monotherapy for at least 4 weeks.Exclude patients use Risperidone in combination with other antipsychotic drugs 30 days prior to enrollment .
Patients are randomly divided into three groups, four weeks reduction groups: the group continue to receive the effective dose of the acute phase for 4 weeks, followed by 8 weeks when the dose is gradually reduced to 1/2 of the original amount of treatment, followed by four weeks when doctors are allowed according to the patient's condition, efficacy and tolerability to decide whetherthe dose is further reduced to 1/4 of the original amount of treatment and to maintain this dose until the end of the study. 26 weeks reduction group: the group continue to receive an effective dose of the acute phase of treatment for 26 weeks, followed by the same manner as in group A the dose is reduced and maintained to the end of treatment research. Continuous dose group: Use the effective dose of acute phase of treatment ,there is no reduction throughout the study period.
The results show that using time to recurrence or relapse rate as the evaluation index, continuing with an effective dose of the acute phase of treatment for 4 weeks and 26 weeks , then reducing the dose, can not effectively reduce the recurrence rate. A group has the highest risk of recurrence which is 31.5%, and the results are very similar to those in Cscernansky reports( risk of recurrence using Risperidone for 28 months is 34%). A long time for maintenance treatment or when continuing without reduction can reduce the risk of recurrence . Kane, etc. Using different doses of haloperidol decanoate maintenance therapy for 1 year ,finds that as the dose increases, relapse rate tends to decrease. The use of long-acting drugs actually ensures that the dose is sustained,which also shows a lower relapse rate which is consistent with the results of this study.
Studies have shown that after acute schizophrenia treatment, continue use of Risperidone in the acute phase of an effective dose without reduction maintenance therapy, which has the lowest risk of relapse and improves the symptoms further, and does not increase relatively common weight gain, extrapyramidal symptoms and menstrual disorders and so on significantly. These results indicate that, in order to reduce the relapse rate in patients with acute schizophrenia, the use of an effective dose of Risperidone maintenance treatment ≥6 months is necessary.
The multicenter open randomized controlled study is participated by the National 19 psychiatric hospitals or psychiatric general hospitals, Objects of study are patients in line with the American Diagnostic and Statistical Manual Mental Disorders 4th edition (DSM-Ⅳ) schizophrenia diagnostic criteria , with previous episodes at least once,the total treatment time from this episode to join to the group, is ≤5 months,and they have been completed acute treatment and the symptoms are under control, they have been using fixed-dose Risperidone monotherapy for at least 4 weeks.Exclude patients use Risperidone in combination with other antipsychotic drugs 30 days prior to enrollment .
Patients are randomly divided into three groups, four weeks reduction groups: the group continue to receive the effective dose of the acute phase for 4 weeks, followed by 8 weeks when the dose is gradually reduced to 1/2 of the original amount of treatment, followed by four weeks when doctors are allowed according to the patient's condition, efficacy and tolerability to decide whetherthe dose is further reduced to 1/4 of the original amount of treatment and to maintain this dose until the end of the study. 26 weeks reduction group: the group continue to receive an effective dose of the acute phase of treatment for 26 weeks, followed by the same manner as in group A the dose is reduced and maintained to the end of treatment research. Continuous dose group: Use the effective dose of acute phase of treatment ,there is no reduction throughout the study period.
The results show that using time to recurrence or relapse rate as the evaluation index, continuing with an effective dose of the acute phase of treatment for 4 weeks and 26 weeks , then reducing the dose, can not effectively reduce the recurrence rate. A group has the highest risk of recurrence which is 31.5%, and the results are very similar to those in Cscernansky reports( risk of recurrence using Risperidone for 28 months is 34%). A long time for maintenance treatment or when continuing without reduction can reduce the risk of recurrence . Kane, etc. Using different doses of haloperidol decanoate maintenance therapy for 1 year ,finds that as the dose increases, relapse rate tends to decrease. The use of long-acting drugs actually ensures that the dose is sustained,which also shows a lower relapse rate which is consistent with the results of this study.
Studies have shown that after acute schizophrenia treatment, continue use of Risperidone in the acute phase of an effective dose without reduction maintenance therapy, which has the lowest risk of relapse and improves the symptoms further, and does not increase relatively common weight gain, extrapyramidal symptoms and menstrual disorders and so on significantly. These results indicate that, in order to reduce the relapse rate in patients with acute schizophrenia, the use of an effective dose of Risperidone maintenance treatment ≥6 months is necessary.
Risperidone is a novel antipsychotic introduced for the treatment of acute and chronic
schizophrenia. It has a balanced serotonin 5-HT2 and dopamine D2 receptor antagonist
activity. While the anti-D2 activity may relate to the antipsychotic potency of neuroleptic
agents, an antidepressive efficacy of substances with anti-5-HT2 activity has been
suggested. Risperidone, therefore, has therapeutic action on both positive and negative
symptoms of schizophrenia and produces significantly fewer side effects especially
extrapyramidal symptoms compared with commonly used pure D2 antagonist antipsychotics.
It also has potential for management of alcohol withdrawal and cocaine addiction.
For the treatment of schizophrenia in adults and in adolescents, ages 13 to 17, and for the short-term treatment of manic or mixed episodes of bipolar I disorder in children and adolescents ages 10 to 17. May also be used to manage symptoms of inappropria
Risperidone has been used:
- to study its effects on bone formation and differentiation
- to investigate the relationship between risperidone (RIS) dosages and RIS plasma levels in autism spectrum disorder (ASD) pediatric patients
- to reverse induced schizophrenia-like behavior in mice
ChEBI: A member of the class of pyridopyrimidines that is 2-methyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one carrying an additional 2-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl group at position 2.
Risperidone (Risperdal, a benzisoxazole)has the structural features of a hybrid molecule between abutyrophenone antipsychotic and a trazodone-like antidepressant.Its superior side effects profile (compared withhaloperidol) at dosage of 6 mg/d or less and the lower riskof tardive dyskinesia have contributed to its very widespreaduse. It benefited refractory psychotic patients, withparkinsonism controlled at one tenth the dose of antiparkinsoniandrugs used with haloperidol.Coexisting anxietyand depressive syndromes were also lessened. It is reportedto decrease the negative (e.g., withdrawal, apathy) as well asthe positive (e.g., delusions, hallucinations) symptoms ofschizophrenia. This is reportedly a consequence of the compound’scombination 5-HT2–D2 receptor antagonistic properties.Overall, the reasons for the decreased EPS and effectiveness against negative symptom are still under investigation.It is an important atypical antipsychotic.Risperidone is metabolized in the liver by CYP2D6 to anactive metabolite, 9-hydroxyrisperidone. Because thismetabolite and risperidone are nearly equipotent, the clinicalefficacy of the drug reflects both compounds.
Risperidone, 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2]pyrimidin-4-one (Risperdal), is awhite to slightly beige powder that is essentially insoluble inwater. Risperidone is also available as a 1-mg/mL oral solutionand as orally disintegrating tablets (Risperdal M-Tab).Risperidone is well absorbed, and peak levels occur about 1hour after administration. The absorption of risperidone is notaffected by food. Risperidone is about 90% bound to albuminand 1-acid glycoprotein, whereas its metabolite 9-hydroxyrisperidoneis bound about 77%. Risperidone is primarilymetabolized in humans to the active metabolite 9-hydroxyrisperidone..The major side effects associated with risperidonetherapy are orthostatic hypotension, dose-related hyperprolactinemia,mild weight gain, EPS, and insomnia.Athigher doses (6 mg/day), risperidone is the atypical antipsychoticthat most closely resembles conventional agents. APET study in a group of individuals with schizophreniashowed that D2 receptor occupancy was dose dependent. Ifthe dose was increased such that D2 receptor occupancy was79% to 85%, the majority of patients developed EPS.Risperidone is associated with increased mortality in elderlypatients with dementia-related psychosis and is not recommendedfor these individuals.23 Risperidone binds with highaffinity at 5-HT2A, 5-HT7, D2, 1, 2, and H1 receptors. Theantipsychotic action of risperidone has been proposed tobe the result of D2 and 5-HT2A antagonism.
Atypical antipsychotic agent that displays 5-HT 2A receptor antagonism. Also displays high affinity at D 2 receptors (K i values are 0.4 and 3.13 nM for 5-HT 2A and D 2 receptors respectively).
Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effect.
Analgesics: increased risk of convulsions with tramadol; enhanced hypotensive and sedative effects with opioids; increased risk of ventricular arrhythmias with methadone - avoid.
Antidepressants: concentration increased by fluoxetine and possibly paroxetine; concentration of tricyclics possibly increased.
Antiepileptics: antagonism, convulsive threshold may be lowered; metabolism accelerated by carbamazepine.
Antimalarials: avoid with artemether with lumefantrine; possible increased risk of ventricular arrhythmias with mefloquine and quinine.
Antipsychotics: possible increased risk of ventricular arrhythmias with other antipsychotics that prolong the QT interval; avoid concomitant use of depot formulations with clozapine (cannot be withdrawn quickly if neutropenia occurs).
Antivirals: ritonavir may increase concentration of risperidone.
Anxiolytics and hypnotics: enhanced sedative effects.
Atomoxetine: increased risk of ventricular arrhythmias.
Beta blockers: possible increased risk of ventricular arrhythmias with sotalol.
Cytotoxics: increased risk of ventricular arrhythmias with arsenic trioxide.
Lithium: increased risk of extra-pyramidal side effects and possible neurotoxicity.
Anaesthetics: enhanced hypotensive effect.
Analgesics: increased risk of convulsions with tramadol; enhanced hypotensive and sedative effects with opioids; increased risk of ventricular arrhythmias with methadone - avoid.
Antidepressants: concentration increased by fluoxetine and possibly paroxetine; concentration of tricyclics possibly increased.
Antiepileptics: antagonism, convulsive threshold may be lowered; metabolism accelerated by carbamazepine.
Antimalarials: avoid with artemether with lumefantrine; possible increased risk of ventricular arrhythmias with mefloquine and quinine.
Antipsychotics: possible increased risk of ventricular arrhythmias with other antipsychotics that prolong the QT interval; avoid concomitant use of depot formulations with clozapine (cannot be withdrawn quickly if neutropenia occurs).
Antivirals: ritonavir may increase concentration of risperidone.
Anxiolytics and hypnotics: enhanced sedative effects.
Atomoxetine: increased risk of ventricular arrhythmias.
Beta blockers: possible increased risk of ventricular arrhythmias with sotalol.
Cytotoxics: increased risk of ventricular arrhythmias with arsenic trioxide.
Lithium: increased risk of extra-pyramidal side effects and possible neurotoxicity.
Risperidone is metabolised in the liver by CYP 2D6
to its main active metabolite, 9-hydroxy-risperidone
(paliperidone), which has a similar pharmacological
activity as risperidone. This hydroxylation is subject to
genetic polymorphism. Oxidative N-dealkylation is a
minor metabolic pathway.
Excretion is mainly in the urine and, to a lesser extent, in the faeces.
Excretion is mainly in the urine and, to a lesser extent, in the faeces.
Preparation Products And Raw materials
Raw materials
- Potassium iodideIsoxazole1,3-Difluorobenzene 4-Pyrimidinol885706-66-1Risperidal iMpurity B3-(2-Chloroethyl)-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one6-Fluoro-3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride3-[2-[4-[(Z)-(2,4-Difluorophenyl)(hydroxyiMino)Methyl]-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-2-Methyl-4H-pyrido[1,2-a]pyriMidin-4-oneRisperidone E-Oxime2,4-Difluorophenyl-(4-piperidinyl)methanoneoxime2,4-Difluorophenyl-(4-piperidinyl)methanone oxime hydrochloride6-Fluoro-3-(4-piperidinyl)-1,2-benzisoxazoleTetracyanoethylene
Preparation Products
Risperdal Supplier
Tel +1-708-310-1919 +1-13798911105
Email sales@invivochem.net
Products Intro
Cas:106266-06-2
ProductName:Risperidone(R-64766;Risperdal)
Brand:InvivoChem | Product Number:V0982 | Purity:≥98%
Cas:106266-06-2
ProductName:Risperidone(R-64766;Risperdal)
Brand:InvivoChem | Product Number:V0982 | Purity:≥98%
Tel 800.520.3011 or 781.639.5092
Email chemicals@usbio.net
Products Intro
Cas:
ProductName:Risperidone (3-[2-4-(6-Fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one, R-64766, Belivon, Risperdal)
Cas:
ProductName:Risperidone (3-[2-4-(6-Fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one, R-64766, Belivon, Risperdal)
Tel 866-930-6790
Email info@adooq.com
Products Intro
Cas:106266-06-2
ProductName:Risperidone (Risperdal)
Purity: 98% | Package: 5mg;10mg;25mg;50mg;100mg;250mg;500mg;1g
Cas:106266-06-2
ProductName:Risperidone (Risperdal)
Purity: 98% | Package: 5mg;10mg;25mg;50mg;100mg;250mg;500mg;1g
Tel 1-800-520-3011
Email sales@advtechind.com
Products Intro
Cas:
ProductName:Risperidone (3-[2-4-(6-Fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one, R-64766, Belivon, Risperdal)
Cas:
ProductName:Risperidone (3-[2-4-(6-Fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one, R-64766, Belivon, Risperdal)
Related Product Information
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine
