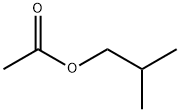
Isobutyl acetate, also known as n-propyl acetate, is the esterfication product between acetic acid and 2-butanol. It is a water-white liquid with flavor of soft fruit ester. It is slightly soluble in water, miscible with ethanol, ethyl ether as well as many other kinds of organic solvent including alcohol, ether and hydrocarbon. It can be mainly used as the diluent of nitro-lacquer and perchlorethylene paint and the solvents of nitrocellulose and lacquer as well as the substitution solvent of butyl acetate and methyl isobutyl ketone. It can also be used as a component of the flavoring agent. It can also be used as the diluent of plastic printing paste and the extraction agent in the pharmaceutical industry.
Sec-butyl acetate has excellent capability of dissolving many substances. It can be industrially applied as the solvent for manufacturing of nitrocellulose paint, acrylic paint, polyurethane paint, etc., these paints can also be used as an aircraft wing paint, artificial leather coatings and automotive coatings. Its dissolving capability is similar as n-butyl acetate and isobutyl acetate. In the coating formulation, it can be widely adopted for substitution of n-butyl acetate and isobutyl acetate. In metallic paints, people can apply butyl acetate to dissolve the cellulose acetate butyrate to obtain 15% to 20% solution.
The above information is edited by the chemicalbook of Dai Xiongfeng.
It is a water-white liquid with soft fruit ester flavor. It is miscible with a variety of organic solvents such as alcohols, ethers and hydrocarbons.
Isobutyl Acetate can be used as organic solvent, the solvent of nitrocellulose and lacquer, extraction agent, dehydrating agents. It can also be applied to collodion, nitrocellulose, varnishes, leather, pharmaceuticals, plastics and perfume industry.
It can be determined according to the method 1 in ester assay (OT-18). The amount of the sample for taking is 1g. The equivalency factor (e) for the calculation can be taken as 58.08. Alternatively, people can apply non-polar column method via gas chromatography (GT-10-4) for the determination.
The above information is edited by the chemicalbook of Dai Xiongfeng.
LD50: 13400 mg/kg (rat, oral).
GRAS (FEMA).
FEMA (mg/kg): soft drinks: 11; cold drink: 16; Confectionery: 36; Bakery: 35; pudding class: 170; gum: 860; coating: 5.5.
Take appropriate amount as limit (FDA§172.515,2000).
It can be obtained via the esterfication between iso-butanol and acetic anhydride in the presence of sulfuric acid. Mix the acetic anhydride and iso-butanol solution followed by adding drop wise of sulfuric acid. Heat for reflux of 5-6 h after a bit cooling, wash the refluxed liquid with water for 2-3 times. Use sodium carbonate for neutralizing with sodium carbonate, wash with water until neutralized, dry over calcium chloride with vacuum distillation in oil bath to derive the finished products.
Isobutyl acetate has a fruity (currant-pear), floral (hyacinth-rose)
odor and a characteristic ether-like, slightly bitter flavor. May be
prepared by direct esterification of isobutyl alcohol with acetic
acid.
Isobutyl acetate, also known as 2-methylpropyl ethanoate (IUPAC name) or β-methylpropyl
acetate, is a common solvent. It is produced from the esterifi cation of isobutanol with acetic
acid. It is used as a solvent for lacquer and nitrocellulose. Like many esters, it has a fruity
or fl oral smell at low concentrations and occurs naturally in raspberries, pears, and other
plants. At higher concentrations, the odor can be unpleasant and may cause symptoms of
CNS depression, such as nausea, dizziness, and headache
Isobutyl acetate is a colorless liquid with a fruit flavor. Isobutyl acetate is moisture sensitive, incompatible with ignition sources, moisture, excess heat, strong oxidizing agents, and strong bases; on decomposition, it releases carbon monoxide and carbon dioxide. It is used for nitrification fiber and paint solvents, chemical reagents, and modulation spices
Butyl acetates are colorless or yellowish
liquids with pleasant, fruity odors. There are 4 isomers.
Colorless liquid with a fruity odor. Experimentally determined detection and recognition odor
threshold concentrations were 1.7 mg/m3 (360 ppbv) and 2.4 mg/m3 (510 ppbv), respectively
(Hellman and Small, 1974).
Reported found in, apple, apricot, banana, currants, guava, grapes, melon, pear, blackcurrant, papaya, pine�apple, strawberry, vinegar, wheat bread, Parmesan and Gruyere cheese, beef fat, beer, cognac, rum, cider, whiskies, sherry, grape
wines, port, olive, cocoa, passion fruit, plum, starfruit, bantu beer, plum and grape brandy, mango, tamarind, apple brandy, figs, plum
wine, litchi, sake, nectarine, naranjilla fruit, Cape gooseberry and Roman chamomile oil.
Isobutyl acetate is used as a solvent and as aflavoring agent.
Isobutyl Acetate is a flavoring agent that is a clear colorless liquid
with a fruity odor resembling banana when diluted. it is soluble in
alcohol, propylene glycol, most fixed oils, and mineral oil, and
slightly soluble in water. it is obtained by synthesis.
ChEBI: The acetate ester of isobutanol.
By direct esterification of isobutyl alcohol with acetic acid.
Isobutyl acetate may be made from methyl isobutyl
ketone. It may also be made by treating isobutanol
with acetic acid in the presence of catalysts. The
Tischenko reaction of acetaldehyde with isobutyraldehyde
yields a mixture of isobutyl acetate with ethyl acetate and
isobutyl isobutyrate.
Taste characteristics at 30 ppm: sweet fruity with a banana tutti-frutti note.
A clear colorless liquid with a fruity odor. Flash point 64°F. Less dense than water (6.2 lb / gal) and insoluble in water. Vapors are heavier than air .
Highly flammable. Insoluble in water.
Isobutyl acetate reacts exothermically with acids to give alcohols and other acids. May react sufficiently exothermically with strong oxidizing acids to ignite the reaction products. Reactions with bases also generate heat. Combination with strong reducing agents (alkali metals and hydrides) generates flammable hydrogen.
Flammable, dangerous fire risk.
Vapors may irritate upper respiratory tract and cause nausea, vomiting, dizziness and loss of consciousness. Liquid irritates eyes and may irritate skin.
Isobutyl acetate is more toxic but less of anirritant than n-butyl acetate. The toxic symp toms include headache, drowsiness, irritationof upper respiratory tract, and anesthesia.A 4-hour exposure to 8000 ppm was lethalto rats. It produced mild to moderate irri tation on rabbits’ skin. The irritation ineyes was also mild to moderate. The LD50oral value in rabbit is within the range4800 mg/kg.
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Flammability and Explosibility
Not classified
Reactivity with Water No reaction; Reactivity with Common Materials: Softens and dissolves many types of plastics; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
n-Butyl acetate is an important solvent
in the production of lacquers, leather and airplane dopes,
and perfumes. It is used as a solvent and gasoline additive.
sec-Butyl acetate is used as a widely used solvent for
nitrocellulose, nail enamels and many different purposes.
tert-Butyl acetate is common industrial solvent used in the
making of lacquers, artificial leather, airplane dope, perfume; and as a food additive. Isobutyl acetate is used as a
solvent and in perfumes and artificial flavoring materials
A product of whiskey fermentation (quoted, Verschueren, 1983). Isobutyl acetate was
identified as a volatile constituent released by fresh coffee beans (Coffea canephora variety
Robusta) at different stages of ripeness (Mathieu et al., 1998).
Chemical/Physical. Slowly hydrolyzes in water forming 2-methylpropanol and acetic acid.
At an influent concentration of 1,000 mg/L, treatment with GAC resulted in an effluent
concentration of 180 mg/L. The adsorbability of the carbon used was 164 mg/g carbon (Guisti et
al., 1974).
UN1123 Butyl acetates, Hazard Class: 3; Labels:
3—Flammable liquid.
All butyl acetates are incompatible with
nitrates, strong oxidizers; strong alkalies; strong acids.
Butyl acetates may form explosive mixture with air; reacts
with water, on standing, to form acetic acid and n-butyl
alcohol. Violent reaction with strong oxidizers and
potassium-tert-butoxide. Dissolves rubber, many plastics,
resins and some coatings. May accumulate static electrical
charges, and may cause ignition of its vapors
The initial threshold screening level (ITSL) for all isomers of butyl acetate is 2400 μg/m3 with eight-hour averaging time.
Dissolve or mix the material
with a combustible solvent and burn in a chemical
incinerator equipped with an afterburner and scrubber.
All federal, state, and local environmental regulations
must be observed.