Caionic surfactants
Cationic surfactant can be dissociated in water, having a molecular structure of surface activity and partially exhibit hydrophobic cationic behavior. At as early as 1896, F. Kraft et al has found the hydrochloride of hexadecylamine having the foaming properties of soaps, but it was not until after 1928 before the cationic surfactant had been adopted in the industry such as being used as fabric softeners, antistatic agent, water-repellent agents, dyeing auxiliaries, flotation and germicides. But its consumption amount is lower than the anionic surfactant and non-ionic surfactants and is mainly used in some special applications such as reducing the friction and sterilization effect.
The cationic surfactants can dissociate cation of surface activity in the water with its charge being contrary to an anionic surfactant, thus being often called "inverse soap." In term of its chemical structure, it contains at least one long-chain hydrophobic group and a positively charged hydrophilic group. The hydrophobic group of the long-chain is usually derived from fatty acids or petroleum chemicals. The positive charge of the cationic surfactants is generally carried by the nitrogen atom, alternatively may also be carried by the sulfur and phosphorus atoms. However, among hundreds of cationic surfactants of commercialized value, most of them contain a positively charged nitrogen atom. Thus, fatty amine is an important raw material of cationic surfactant.
The cationic surfactants, similar as other types of surfactant, after being absorbed on the surface or the interface to a certain concentration (the critical micelle concentration, i.e., CMC) will form micelles in solution, thereby reducing the surface tension of the solvent, exhibit surface activity. It has emulsifying, solubilizing, wetting, rinsing and dispersion effects. The rinsing effect of the cationic surfactant is limited with its antibacterial activity and the adhesion affinity to a hard surface being more prominent. In general, hard surface contains negative charge with the positively charged cationic surfactants having a very significant activity on it. The cationic surfactants can be easily absorbed by human skin, hair and teeth. They can be used as sanitizing agent, antiseptic disinfectant, germicides, fungicides, antistatic agents, textile softener, corrosion inhibitor, anti-foaming agent and flotation agents and so on. In cosmetic application, it is mainly used for sterilization, antibacterial agents, hair conditioners, skin softener and anti-caries additives. In the late 1980s, the cationic surfactant can account for about 8% to 10% of the total sales of surfactants.
- Structure:
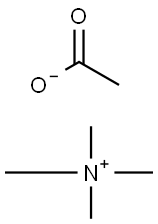
- Chemical Name:Tetramethylammonium acetate
- CAS:10581-12-1
- MF:C6H15NO2
- Structure:
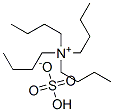
- Chemical Name:Tetrabutylammonium hydrogen sulfate
- CAS:32503-27-8
- MF:C16H37NO4S
- Structure:
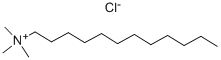
- Chemical Name:Dodecyltrimethylammonium chloride
- CAS:112-00-5
- MF:C15H34ClN
- Structure:
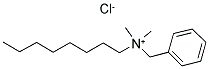
- Chemical Name:Benzalkonium chloride
- CAS:8001-54-5
- MF:C17H30ClN
- Structure:
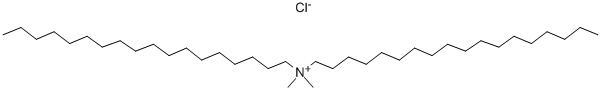
- Chemical Name:Dioctadecyl dimethyl ammonium chloride
- CAS:107-64-2
- MF:C38H80ClN
- Structure:
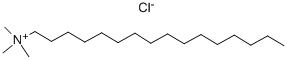
- Chemical Name:N-Hexadecyltrimethylammonium chloride
- CAS:112-02-7
- MF:C19H42ClN
- Structure:
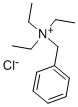
- Chemical Name:Benzyltriethylammonium chloride
- CAS:56-37-1
- MF:C13H22ClN
- Structure:
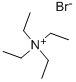
- Chemical Name:Tetraethylammonium bromide
- CAS:71-91-0
- MF:C8H20BrN
- Structure:
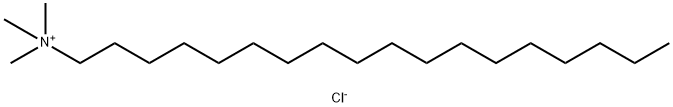
- Chemical Name:Trimethylstearylammonium Chloride
- CAS:112-03-8
- MF:C21H46ClN
- Structure:
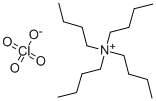
- Chemical Name:Tetrabutylammonium perchlorate
- CAS:1923-70-2
- MF:C16H36ClNO4
- Structure:
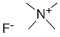
- Chemical Name:Tetramethylammonium fluoride
- CAS:373-68-2
- MF:C4H12FN
- Structure:
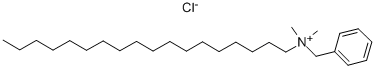
- Chemical Name:Benzyldimethylstearylammonium Chloride
- CAS:122-19-0
- MF:C27H50ClN
- Structure:
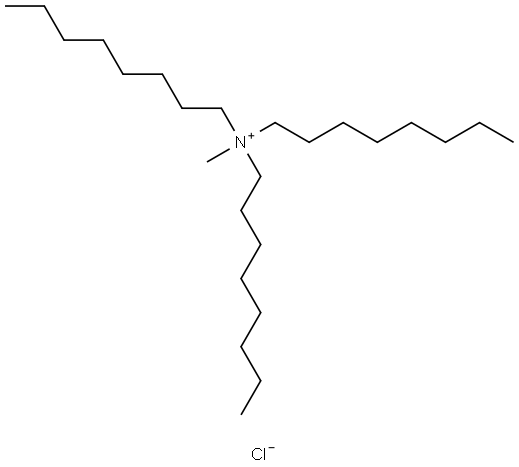
- Chemical Name:Methyl trioctyl ammonium chloride
- CAS:5137-55-3
- MF:C25H54N.Cl
- Structure:
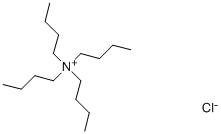
- Chemical Name:Tetrabutyl ammonium chloride
- CAS:1112-67-0
- MF:C16H36ClN
- Structure:
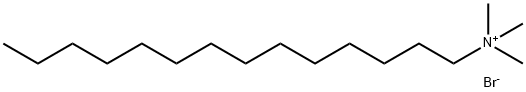
- Chemical Name:Tetradecyltrimethylammonium bromide
- CAS:1119-97-7
- MF:C17H38N.Br
- Structure:
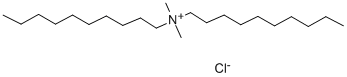
- Chemical Name:Didecyl dimethyl ammonium chloride
- CAS:7173-51-5
- MF:C22H48ClN
- Structure:

- Chemical Name:Tetramethylammonium iodide
- CAS:75-58-1
- MF:C4H12IN
- Structure:

- Chemical Name:Dodecyltrimethylammonium Bromide
- CAS:1119-94-4
- MF:C15H34BrN
- Chemical Name:Polyquaternium-7
- CAS:108464-53-5
- MF:(C8H16ClN)n.(C3H5NO)m
- Structure:
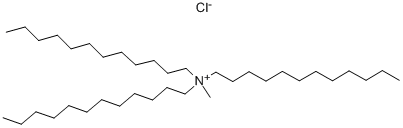
- Chemical Name:Tridodecyl methyl ammonium chloride
- CAS:7173-54-8
- MF:C37H78ClN
- Chemical Name:Benzalkonium chloride
- CAS:85409-22-9
- MF:C9H13NRCl
- Structure:
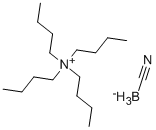
- Chemical Name:Tetrabutylammonium cyanoborohydride
- CAS:43064-96-6
- MF:C17H39BN2
- Chemical Name:G-271(TM)
- CAS:61791-34-2
- MF:
- Structure:
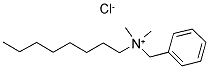
- Chemical Name:BENZALKONIUM CHLORIDE
- CAS:68424-85-1
- MF:C17H30ClN
- Chemical Name:alkyl dimethyl benzyl ammonium chloride (n=14)
- CAS:
- MF:
- Structure:
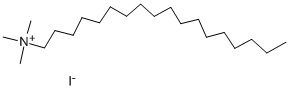
- Chemical Name:Behenyl Trimethyl Ammonium Chloride
- CAS:4292-25-5
- MF:C22H45(CH3)3NCl
- Chemical Name:Flocculant ST
- CAS:
- MF:
- Structure:
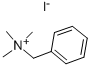
- Chemical Name:Benzyltrimethylammonium iodide
- CAS:4525-46-6
- MF:C10H16IN
- Chemical Name:l-Alanine, N-coco acyl derivs., sodium salts
- CAS:90170-45-9
- MF:
- Structure:
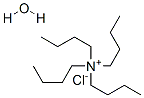
- Chemical Name:Tetrabutylammonium chloride monohydrate
- CAS:88641-55-8
- MF:C16H36N.Cl.H2O
- Structure:
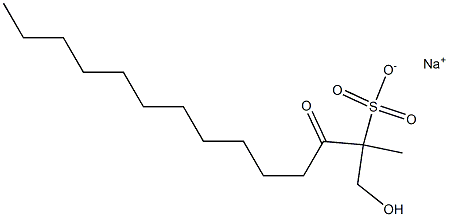
- Chemical Name:sodium lauroyl methyl isethionate
- CAS:928663-45-0
- MF:C15H30O5S.Na
- Chemical Name:DICOCO DIMETHYL AMMONIUM CHLORIDE
- CAS:61789-77-3
- MF:
- Structure:

- Chemical Name:Octadecylamine N-oleoyl Sarcosinate
- CAS:
- MF:C39H78N2O3
- Structure:
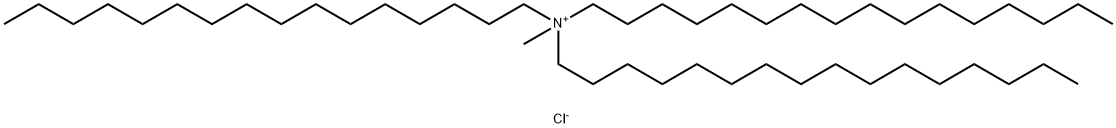
- Chemical Name:N,N-Dihexadecyl-N-methyl-1-hexadecanaminium chloride
- CAS:52467-63-7
- MF:C49H102ClN
- Structure:
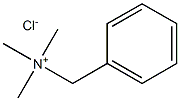
- Chemical Name:benzyltrimeehyl ammonium chloride
- CAS:
- MF:C10H16ClN
- Structure:
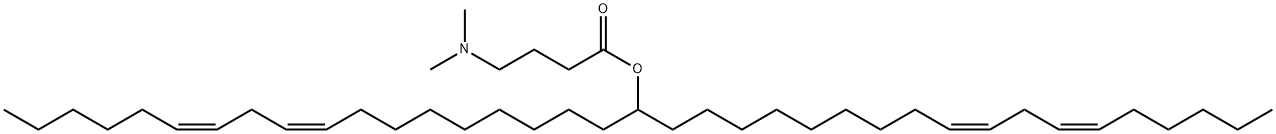
- Chemical Name:DLin-MC3-DMA
- CAS:1224606-06-7
- MF:C43H79NO2
- Structure:
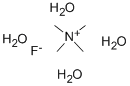
- Chemical Name:Tetramethylammonium fluoride tetrahydrate
- CAS:17787-40-5
- MF:C4H20FNO4
- Structure:
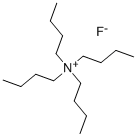
- Chemical Name:Tetrabutylammonium fluoride
- CAS:429-41-4
- MF:C16H36FN
- Structure:
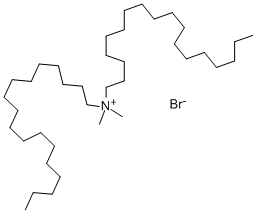
- Chemical Name:Dimethyldioctadecylammonium bromide
- CAS:3700-67-2
- MF:C38H80BrN
- Structure:
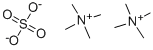
- Chemical Name:Tetramethylammonium sulfate
- CAS:14190-16-0
- MF:C8H24N2O4S
- Structure:
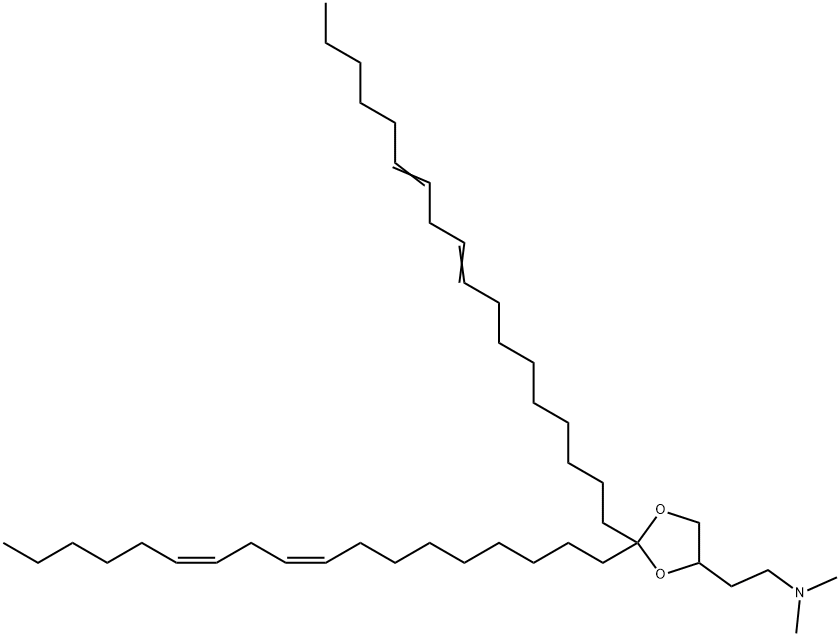
- Chemical Name:DLin-KC2-DMA
- CAS:1190197-97-7
- MF:C43H79NO2
- Structure:
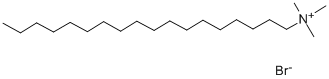
- Chemical Name:Octadecy trimethyl ammonium bromide
- CAS:1120-02-1
- MF:C21H46BrN