Antimalarial drugs
Antimalarials are able to kill or inhibit the malaria parasite and the process of plasmodium propagation, being drugs for the prevention and treatment of malaria. On as early as the 1st, 2nd century (AD), there had been already records about the antimalarial effect of antifebrile dichroa in "Shen Nong's Herbal Classic."
The earliest applied natural antimalarial drug, quinine, was the antimalarial component in the cinchona bark used by Indian for the treatment of fever. In 1907, Rabe had clarified its chemical structure. Subsequently, people had been actively engaged in the research of synthesizing antimalarial drugs. In 1926, Schulemann had for the first time synthesized the artificial antimalarial-plasmochin, also known as pamaquine. Atebrine and chloroquine have also successively come out. In 1944, Woodward had successfully performed de novo synthesis of quinine and confirmed its structure. During the World War II, people had successfully synthesized primaquine and pentaquine to replace the pamaquine; starting from the antagonism of biochemical metabolism, people had successfully synthesized proguanil and pyrimethamine.
In early 1960, the emergence of the resistance of plasmodium to chloroquine had set off a new climax to identify the novel antimalarial drugs. Because of the foreign-depth study of the structure-activity relationship of quinine, people had developed novel antimalarial drugs, quinoline-methanols with the representative being the meqloquine. Starting from the study of the structure-activity relationship of the 4-amino-quinoline drugs, people had synthesized amodiaquine-type or hydroxypiperaquine-type drugs such as bis-pyroquilon (M6407), pyracrine and pyronaridine which have bilateral side chain of pyrrolidine and methyl aminophenol. They not only have lower cardiac toxicity than chloroquine and also have varying degrees of anti-arrhythmia effects.
Chinese scientists have discovered Radix dichroa), Hydrangea umbellate and Brucea javanicaL.Merr from Chinese herbs. Through analysis, people have clarified the chemical structure of the Febrifugine. At early 1970s, people have further isolated artemisinin from Artemisia annua. Animal experiments and clinical observations had confirmed that its antimalarial effects are more excellent than chloroquine and it can be used to rescue dangerous type of malaria. The analysis results of chemical structure have demonstrated that it is a kind of sesquiterpene containing the peroxide bridge, being a totally new type in the anti-malarial drugs. People have realized the de novo total synthesis of this kind of peroxide bridges sesquiterpene lactones in 1982. Chinese scholars have also found another kind of sesquiterpene containing the peroxide bridge, yingzhaosu A, from the Artabotrys hexapetalus. Artemisinin and its derivatives also have significant anti-schistosome effect.
In addition, the antibiotic clindamycin and tetracyclines also have antimalarial effect. However, due to the slow effect, they are generally not used alone. There are also clindamycin which can be used in combination with quinine to treat falciparum malaria and so on.
- Structure:
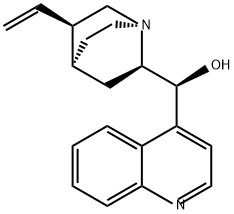
- Chemical Name:Cinchonine
- CAS:118-10-5
- MF:C19H22N2O
- Structure:
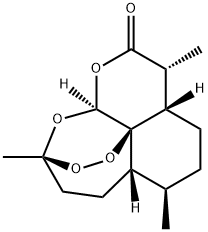
- Chemical Name:Artemisinin
- CAS:63968-64-9
- MF:C15H22O5
- Structure:
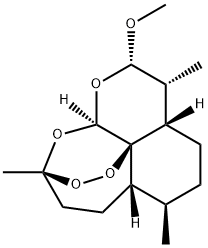
- Chemical Name:Artemether
- CAS:71963-77-4
- MF:C16H26O5
- Structure:
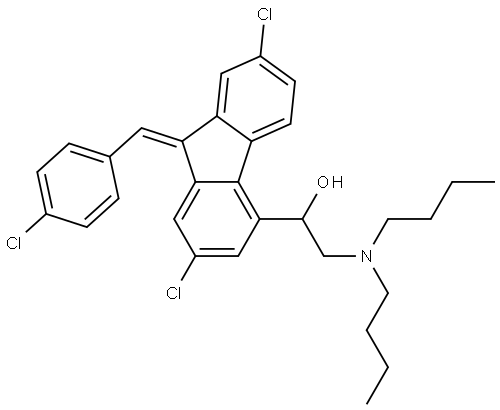
- Chemical Name:Lumefantrine
- CAS:82186-77-4
- MF:C30H32Cl3NO
- Structure:
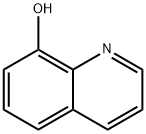
- Chemical Name:8-Hydroxyquinoline
- CAS:148-24-3
- MF:C9H7NO
- Structure:
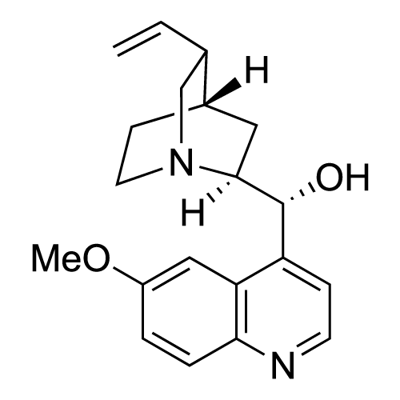
- Chemical Name:Quinine
- CAS:130-95-0
- MF:C20H24N2O2
- Structure:
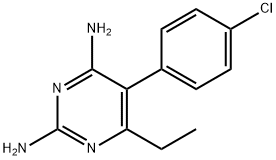
- Chemical Name:Pyrimethamine
- CAS:58-14-0
- MF:C12H13ClN4
- Structure:
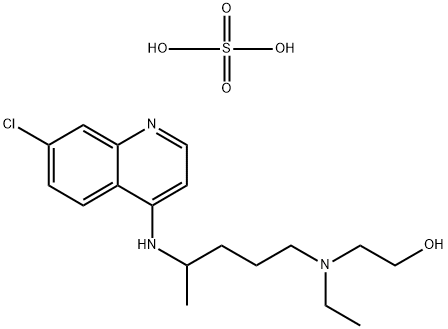
- Chemical Name:Hydroxychloroquine sulfate
- CAS:747-36-4
- MF:C18H28ClN3O5S
- Structure:
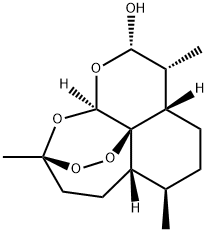
- Chemical Name:DHQHS 2
- CAS:71939-50-9
- MF:C15H24O5
- Structure:
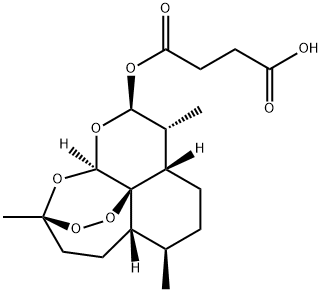
- Chemical Name:Artesunate
- CAS:88495-63-0
- MF:C19H28O8
- Structure:
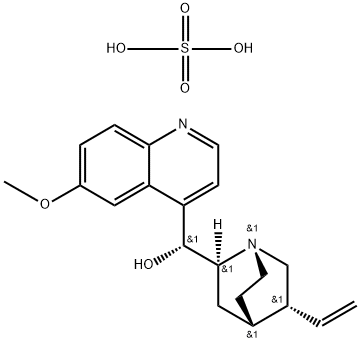
- Chemical Name:Quinine sulfate dihydrate
- CAS:6119-70-6
- MF:C20H26N2O6S
- Structure:
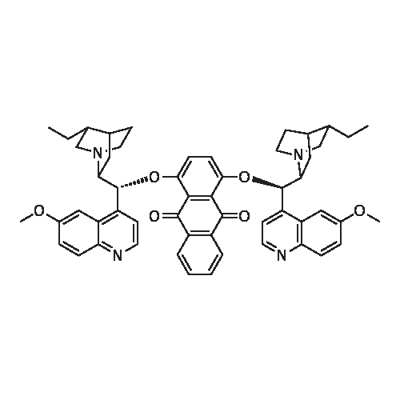
- Chemical Name:HYDROQUININE (ANTHRAQUINONE-1 4-DIYL)
- CAS:176097-24-8
- MF:C54H56N4O6
- Chemical Name:artesunate
- CAS:83507-69-1
- MF:
- Structure:
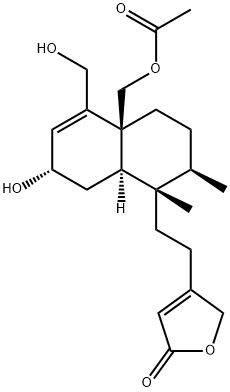
- Chemical Name:4-[2-[(1S,2R,4aS,7S,8aR)-4a-[(Acetyloxy)methyl]-1,2,3,4,4a,7,8,8a-octahydro-7-hydroxy-5-(hydroxymethyl)-1,2-dimethyl-1-naphthalenyl]ethyl]-2(5H)-furanone
- CAS:125675-09-4
- MF:C22H32O6
- Structure:
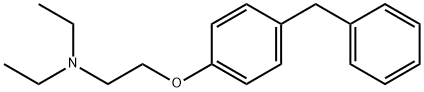
- Chemical Name:Tesmilifene
- CAS:98774-23-3
- MF:C19H25NO
- Structure:
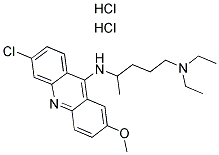
- Chemical Name:QUINACRINE DIHYDROCHLORIDE
- CAS:69-05-6
- MF:C23H32Cl3N3O
- Structure:
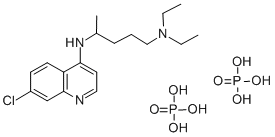
- Chemical Name:Chloroquine diphosphate
- CAS:50-63-5
- MF:C18H32ClN3O8P2
- Structure:
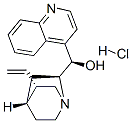
- Chemical Name:CINCHONINE HYDROCHLORIDE
- CAS:5949-11-1
- MF:C19H23ClN2O
- Structure:
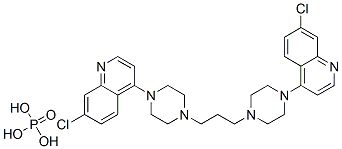
- Chemical Name:4,4'-(1,3-Propanediyldi-4,1-piperazinediyl)bis(7-chloroquinoline) phosphate
- CAS:85547-56-4
- MF:C29H32Cl2N6.H3PO4
- Structure:
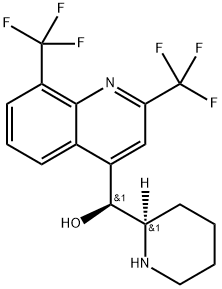
- Chemical Name:MEFLOQUINE
- CAS:53230-10-7
- MF:C17H16F6N2O
- Structure:
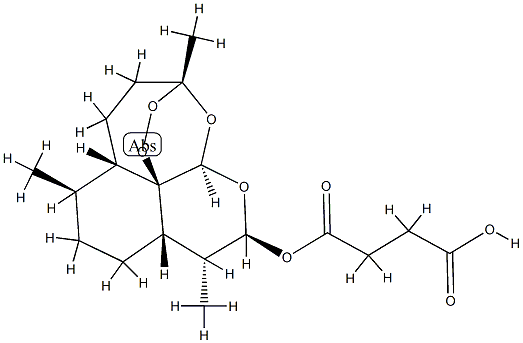
- Chemical Name:Hsdb 7458
- CAS:91487-94-4
- MF:C19H28O8
- Structure:
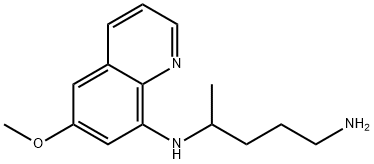
- Chemical Name:Primaquine
- CAS:90-34-6
- MF:C15H21N3O
- Chemical Name:Naphthoquine Phosphate
- CAS:
- MF:
- Structure:
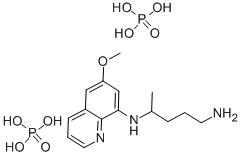
- Chemical Name:Primaquine diphosphate
- CAS:63-45-6
- MF:C15H27N3O9P2
- Structure:
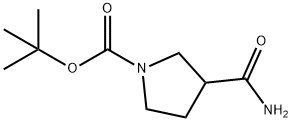
- Chemical Name:3-Aminocarbonyl-1-Boc-pyrrolidine
- CAS:122684-34-8
- MF:C10H18N2O3
- Structure:
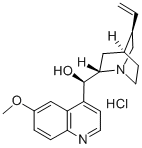
- Chemical Name:Quinine hydrochloride
- CAS:130-89-2
- MF:C20H25ClN2O2
- Structure:
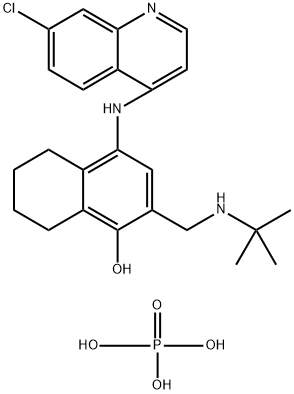
- Chemical Name:NAPHTHOQUINE PHOSPHATE
- CAS:173531-58-3
- MF:C24H31ClN3O5P
- Structure:
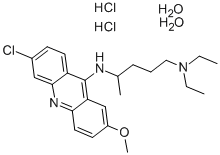
- Chemical Name:Mepacrine hydrochloride
- CAS:6151-30-0
- MF:C23H36Cl3N3O3
- Structure:
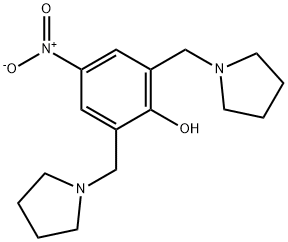
- Chemical Name:Malaridine
- CAS:121836-29-1
- MF:C16H23N3O3
- Structure:
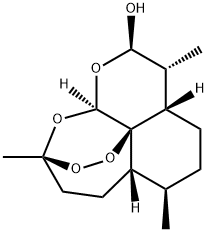
- Chemical Name:alpha-Dihydroartemisinin
- CAS:81496-81-3
- MF:C15H24O5
- Structure:
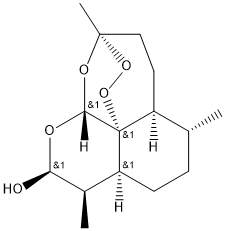
- Chemical Name:Dihydroartemisinin
- CAS:81496-82-4
- MF:C15H24O5
- Structure:
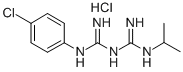
- Chemical Name:Chlorguanide Hydrochloride
- CAS:637-32-1
- MF:C11H17Cl2N5
- Structure:
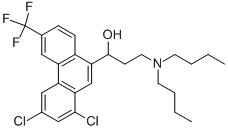
- Chemical Name:HALOFANTRINE
- CAS:69756-53-2
- MF:C26H30Cl2F3NO
- Structure:
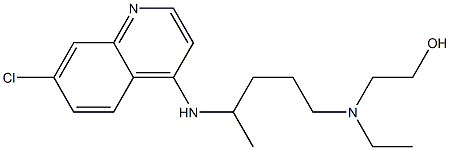
- Chemical Name:Hydroxychloroquine
- CAS:118-42-3
- MF:C18H26ClN3O
- Structure:
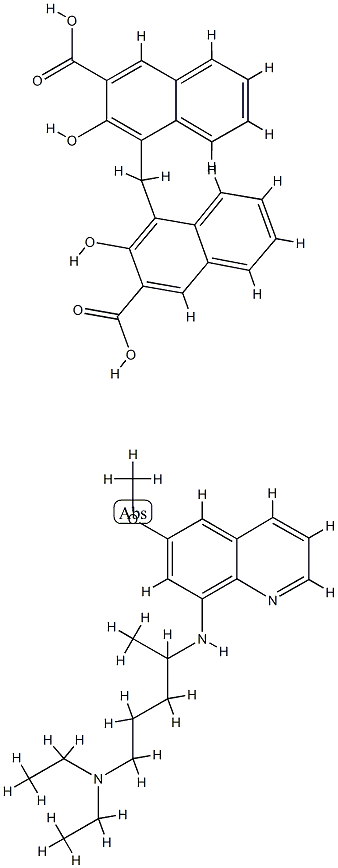
- Chemical Name:pamaquine
- CAS:635-05-2
- MF:C42H45N3O7
- Structure:
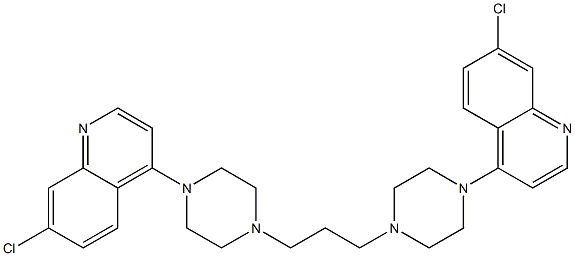
- Chemical Name:Piperaquine
- CAS:83764-65-2
- MF:C29H32Cl2N6
- Structure:
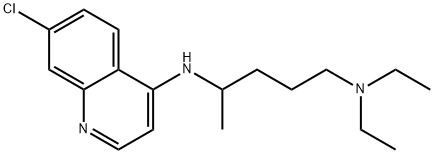
- Chemical Name:CHLOROQUINE
- CAS:54-05-7
- MF:C18H26ClN3
- Structure:
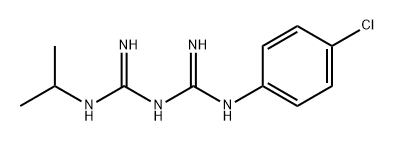
- Chemical Name:N-(4-CHLOROPHENYL)-N'-(ISOPROPYL)-IMIDODICARBONIMIDIC DIAMIDE
- CAS:500-92-5
- MF:C11H16ClN5
- Structure:
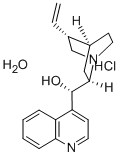
- Chemical Name:CINCHONINE MONOHYDROCHLORIDE HYDRATE 9&
- CAS:312695-48-0
- MF:C19H27ClN2O3
- Structure:
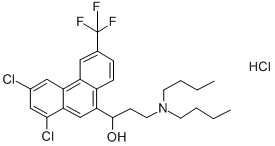
- Chemical Name:Halofantrine hydrochloride
- CAS:36167-63-2
- MF:C26H30Cl2F3NO.ClH
- Chemical Name:Lumefrantrine
- CAS:
- MF:
- Structure:
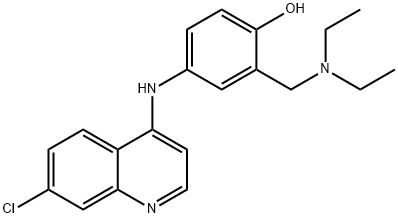
- Chemical Name:Amodiaquine
- CAS:86-42-0
- MF:C20H22ClN3O
- Structure:
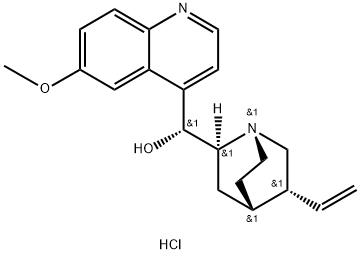
- Chemical Name:Quinine dihydrochloride
- CAS:60-93-5
- MF:C20H25ClN2O2
- Structure:
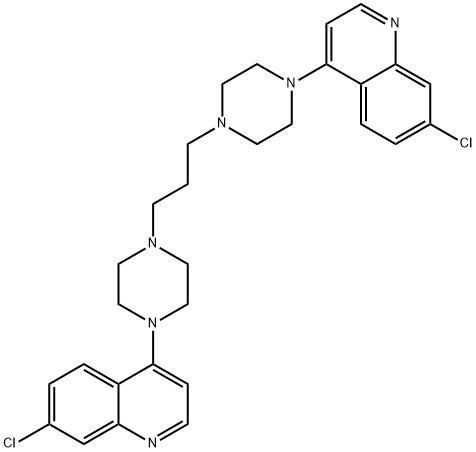
- Chemical Name:Piperaquinoline
- CAS:4085-31-8
- MF:C29H32Cl2N6