Nitrile compound
Nitrile compound is an important chemical raw material and is widely used in the manufacturing of drugs, synthetic fibers and plastics as well as being used in industries of electroplating, steel hardening and mineral processing. Hydrocyanic acid and its salts mainly used for electroplating, mining (extraction of gold and silver), manufacturing of various kinds of resins and synthetic nitrile compound. It sometimes can also be used for warehouse and cabin smoking deratization. Acrylonitrile and methacrylonitrile both belong to an important raw material of synthetic fibers, synthetic rubber and plastics. In addition, in some plants and fruits nucleolus, such as bitter almond, loquat kernels, peach, cassava, ginkgo, all contain cyanide. After intake of excessive amount can occur poisoning, even death of human, especially for children.
The toxicity of nitrile compounds and cyanide for animals vary greatly for different species. Dog and guinea pig are mostly sensitive, followed by mice and rabbits, and the rats are the most insensitive rats. Human sensitivity is moderate.
Organic nitrile compound is a relative good product for being used in perfumery; they may have the following general formula: R-CN, R-O-CN.
Nitrile compound has strong aroma, similar to the corresponding aldehyde, but sharper and more intense than aldehyde.
The most important property of nitrile compound is that it is relatively stable in weakly alkaline medium (such as soaps, detergents and other products) more stable. Therefore, using corresponding nitriles for replacing aldehydes in such products can increase the stability of the flavor.
Inorganic hydrogen cyanide is a highly toxic substance. But for organic cyanide compounds, except benzene acetonitrile that is slightly irritating, so far there is no evidence that higher molecular weight nitrile compounds are toxic and irritating.
Acetonitrile, also known as methyl cyanide, is colorless liquid with aromatic odor and the boiling point being 81.6 ℃. It can be used for organic synthesis and the manufacturing of pharmaceutical and perfume. It belongs to middle-class toxic drug. Mouse oral LD50 is 200 ~ 453.2mg / kg, 2-hour inhalation LC50 is 3,860 ~ 9,651mg / m3. For mice being subject to subcutaneously injection of acetonitrile for 0.01ml /g (2.5% acetonitrile), after two hours, the plasma SCN- content increased and reaches peak after 7 hours with no significant accumulation effect. Human olfactory threshold is 67.2mg / m3. Upon short-term inhalation at 840mg / m3, nasal and throat irritation occur; 840mg / m3 or more can cause nausea, vomiting, chest pain, abdominal pain and vascular changes. This product has been reported of causing occupational poisoning.
- Structure:
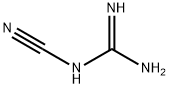
- Chemical Name:Dicyandiamide
- CAS:461-58-5
- MF:C2H4N4
- Structure:

- Chemical Name:Malononitrile
- CAS:109-77-3
- MF:C3H2N2
- Structure:
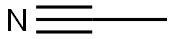
- Chemical Name:Acetonitrile
- CAS:75-05-8
- MF:C2H3N
- Structure:
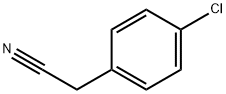
- Chemical Name:4-Chlorobenzyl cyanide
- CAS:140-53-4
- MF:C8H6ClN
- Structure:
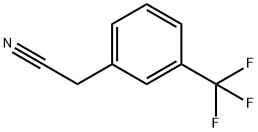
- Chemical Name:3-Trifluoromethylbenzylcyanide
- CAS:2338-76-3
- MF:C9H6F3N
- Structure:
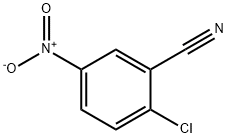
- Chemical Name:2-Chloro-5-nitrobenzonitrile
- CAS:16588-02-6
- MF:C7H3ClN2O2
- Structure:
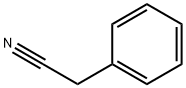
- Chemical Name:Benzeneacetonitrile
- CAS:140-29-4
- MF:C8H7N
- Structure:
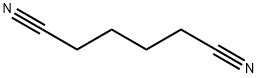
- Chemical Name:Adiponitrile
- CAS:111-69-3
- MF:C6H8N2
- Structure:
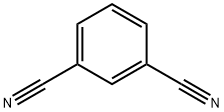
- Chemical Name:1,3-Dicyanobenzene
- CAS:626-17-5
- MF:C8H4N2
- Structure:
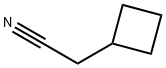
- Chemical Name:CYCLOBUTYLACETONITRILE
- CAS:4426-03-3
- MF:C6H9N
- Structure:
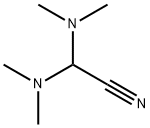
- Chemical Name:bis(dimethylamino)acetonitrile
- CAS:2214-82-6
- MF:C6H13N3
- Structure:
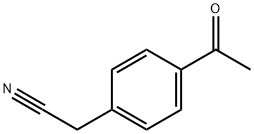
- Chemical Name:4-acetylphenylacetonitrile
- CAS:10266-42-9
- MF:C10H9NO
- Structure:
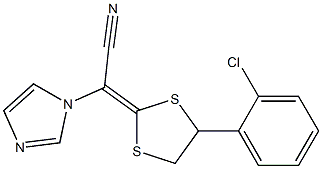
- Chemical Name:Lanoconazole
- CAS:126509-69-1
- MF:C14H10ClN3S2
- Structure:
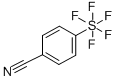
- Chemical Name:4-(Pentafluorothio)benzonitrile
- CAS:401892-85-1
- MF:C7H4F5NS
- Structure:
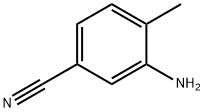
- Chemical Name:3-Amino-4-methylbenzonitrile
- CAS:60710-80-7
- MF:C8H8N2
- Structure:
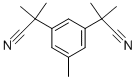
- Chemical Name:3,5-Bis(2-cyanoprop-2-yl)toluene
- CAS:120511-72-0
- MF:C15H18N2
- Structure:
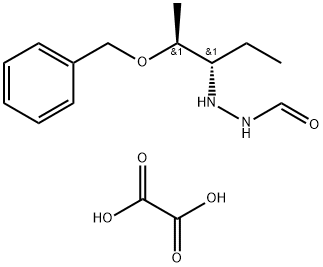
- Chemical Name:N'-((2S,3S)-2-(Benzyloxy)pentan-3-yl)formohydrazide oxalate
- CAS:1887197-42-3
- MF:C15H22N2O6
- Structure:
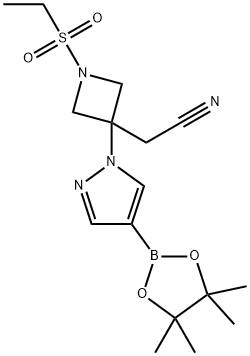
- Chemical Name:3-Azetidineacetonitrile, 1-(ethylsulfonyl)-3-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl]-
- CAS:1919837-50-5
- MF:C16H25BN4O4S
- Structure:
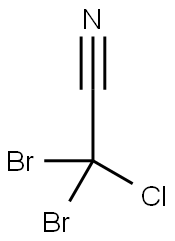
- Chemical Name:2,2-dibromo-2-chloro-acetonitrile
- CAS:144772-39-4
- MF:C2Br2ClN
- Structure:
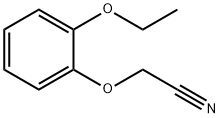
- Chemical Name:(2-ethoxyphenoxy)acetonitrile
- CAS:6781-16-4
- MF:C10H11NO2
- Structure:
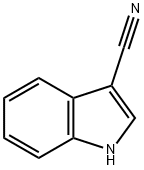
- Chemical Name:3-Cyanoindole
- CAS:5457-28-3
- MF:C9H6N2
- Structure:
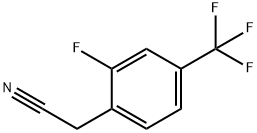
- Chemical Name:2-FLUORO-4-(TRIFLUOROMETHYL)PHENYLACETONITRILE
- CAS:239087-11-7
- MF:C9H5F4N
- Structure:
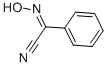
- Chemical Name:2-HYDROXYIMINO-2-PHENYLACETONITRILE
- CAS:825-52-5
- MF:C8H6N2O
- Structure:
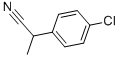
- Chemical Name:4-CHLORO-ALPHA-METHYLPHENYLACETONITRILE
- CAS:2184-88-5
- MF:C9H8ClN
- Chemical Name:N-Desmethyl, N-DesFormylacetonitrileTofacitinib
- CAS:
- MF:
- Structure:
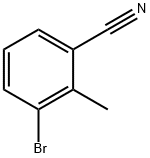
- Chemical Name:3-BROMO-2-METHYLBENZONITRILE
- CAS:52780-15-1
- MF:C8H6BrN
- Structure:
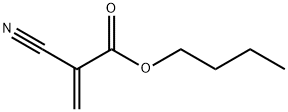
- Chemical Name:enbucrilate
- CAS:6606-65-1
- MF:C8H11NO2
- Structure:
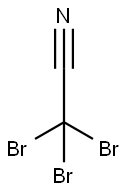
- Chemical Name:Tribromoacetonitrile
- CAS:75519-19-6
- MF:C2Br3N
- Structure:
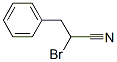
- Chemical Name:2-BROMO-3-PHENYLPROPANENITRILE
- CAS:62448-27-5
- MF:C9H8BrN
- Structure:
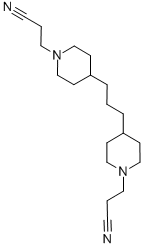
- Chemical Name:4,4'-TRIMETHYLENE-BIS(PIPERIDINOPROPIONITRILE)
- CAS:18136-00-0
- MF:C19H32N4
- Structure:
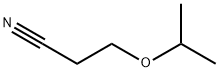
- Chemical Name:BETA-ISOPROPOXYPROPIONITRILE
- CAS:110-47-4
- MF:C6H11NO
- Structure:
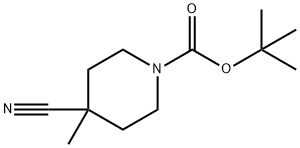
- Chemical Name:tert-Butyl 4-cyano-4-methylpiperidine-1-carboxylate
- CAS:530115-96-9
- MF:C12H20N2O2
- Structure:
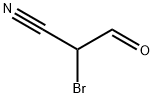
- Chemical Name:2-Bromo-3-oxopropanenitrile
- CAS:97925-42-3
- MF:C3H2BrNO
- Structure:
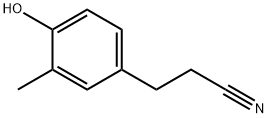
- Chemical Name:Benzenepropanenitrile, 4-hydroxy-3-methyl-
- CAS:22516-99-0
- MF:C10H11NO
- Structure:
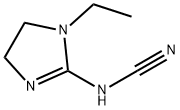
- Chemical Name:1-ethyl-2-cyanoiminoimidazolidine
- CAS:49552-13-8
- MF:C6H10N4
- Structure:
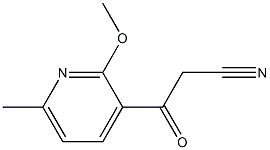
- Chemical Name:3-(2-methoxy-6-methylpyridin-3-yl)-3-oxopropanenitrile
- CAS:1375637-50-5
- MF:C10H10N2O2
- Structure:
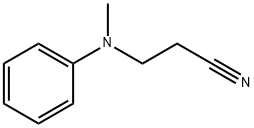
- Chemical Name:N-(2-Cyanoethyl)-N-methylaniline
- CAS:94-34-8
- MF:C10H12N2
- Structure:
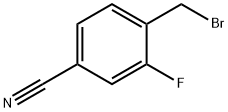
- Chemical Name:2-Fluoro-4-cyanobenzyl bromide
- CAS:105942-09-4
- MF:C8H5BrFN
- Structure:
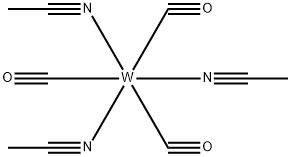
- Chemical Name:Tris(acetonitrile)tricarbonyltungsten
- CAS:16800-47-8
- MF:C9H9N3O3W
- Structure:
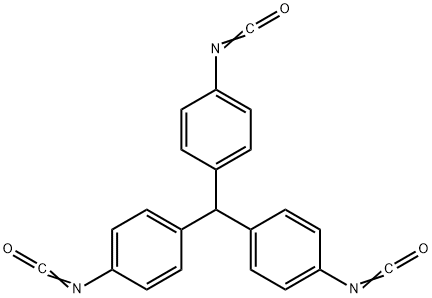
- Chemical Name:Methylidynetri-p-phenylene triisocyanate
- CAS:2422-91-5
- MF:C22H13N3O3
- Structure:
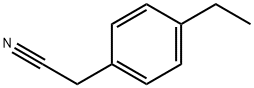
- Chemical Name:4-ETHYLPHENYLACETONITRILE
- CAS:51632-28-1
- MF:C10H11N
- Structure:
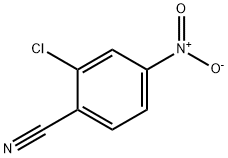
- Chemical Name:2-Chloro-4-nitrobenzonitrile
- CAS:28163-00-0
- MF:C7H3ClN2O2
- Structure:
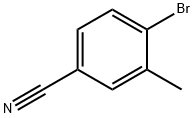
- Chemical Name:4-Bromo-3-methylbenzonitrile
- CAS:41963-20-6
- MF:C8H6BrN
- Structure:
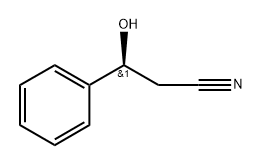
- Chemical Name:132203-26-0
- CAS:132203-26-0
- MF:C9H9NO
- Structure:
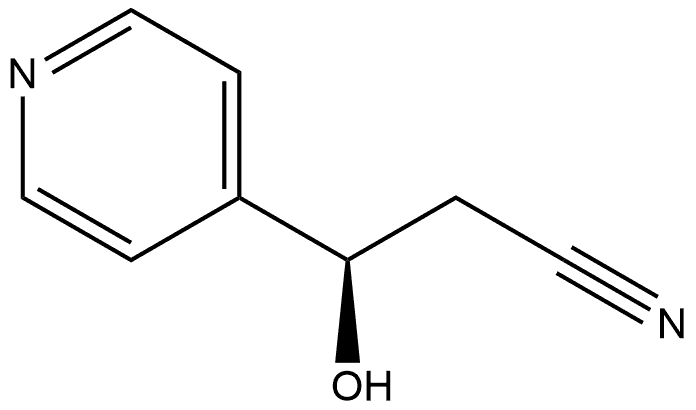
- Chemical Name:(R)-3-hydroxy-3-(pyridin-4-yl)propanenitrile
- CAS:1372452-66-8
- MF:C8H8N2O
- Structure:
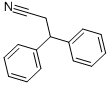
- Chemical Name:3,3-Diphenylpropiononitrile
- CAS:2286-54-6
- MF:C15H13N
- Structure:
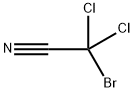
- Chemical Name:Bromodichloroacetonitrile
- CAS:60523-73-1
- MF:C2BrCl2N
- Structure:

- Chemical Name:C10H2Br2N2S2
- CAS:1415139-00-2
- MF:C10H2Br2N2S2
- Structure:
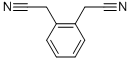
- Chemical Name:1,2-Phenylenediacetonitrile
- CAS:613-23-0
- MF:C10H8N2
- Structure:
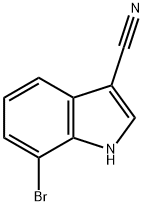
- Chemical Name:1H-Indole-3-carbonitrile, 7-broMo-
- CAS:1043601-50-8
- MF:C9H5BrN2
- Structure:
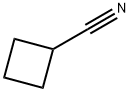
- Chemical Name:Cyclobutanecarbonitrile
- CAS:4426-11-3
- MF:C5H7N
- Structure:
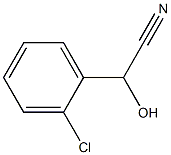
- Chemical Name:2-(2-Chlorophenyl)-2-hydroxyacetonitrile
- CAS:13312-84-0
- MF:C8H6ClNO
- Structure:
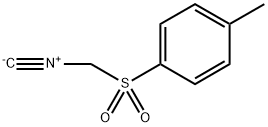
- Chemical Name:Tosylmethyl isocyanide
- CAS:36635-61-7
- MF:C9H9NO2S
- Structure:
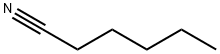
- Chemical Name:HEXANENITRILE
- CAS:628-73-9
- MF:C6H11N
- Structure:
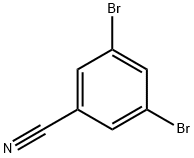
- Chemical Name:3,5-DIBROMOBENZONITRILE
- CAS:97165-77-0
- MF:C7H3Br2N
- Structure:

- Chemical Name:Bicyclo[2.2.1]heptane-1-carbonitrile, 4-amino-
- CAS:2091763-45-8
- MF:C8H12N2
- Structure:
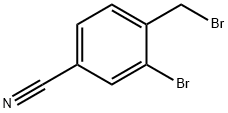
- Chemical Name:3-Bromo-4-(bromomethyl)benzonitrile
- CAS:89892-39-7
- MF:C8H5Br2N
- Structure:
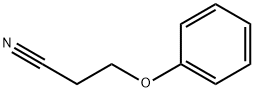
- Chemical Name:3-PHENOXYPROPIONITRILE
- CAS:3055-86-5
- MF:C9H9NO
- Structure:
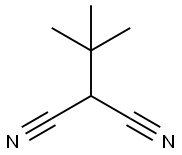
- Chemical Name:TERT-BUTYLMALONONITRILE
- CAS:4210-60-0
- MF:C7H10N2
- Structure:
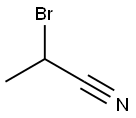
- Chemical Name:2-BROMOPROPIONITRILE
- CAS:19481-82-4
- MF:C3H4BrN
- Structure:
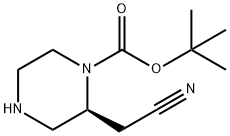
- Chemical Name:tert-butyl (S)-2-(cyanomethyl)piperazine-1-carboxylate
- CAS:1589565-36-5
- MF:C11H19N3O2
- Structure:
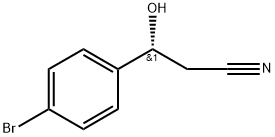
- Chemical Name:(R)-3-(4'-bromophenyl)-3-hydroxypropanenitrile
- CAS:877876-61-4
- MF:C9H8BrNO
- Chemical Name:(S)-2-(9-(pyridin-2-yl)-6-oxaspiro[4.5]decan-9-yl)acetonitrile
- CAS:
- MF:
- Structure:
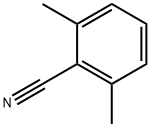
- Chemical Name:2,6-DIMETHYLBENZONITRILE
- CAS:6575-13-9
- MF:C9H9N
- Structure:
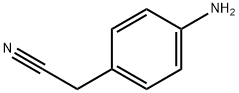
- Chemical Name:4-Aminophenylacetonitrile
- CAS:3544-25-0
- MF:C8H8N2
- Structure:
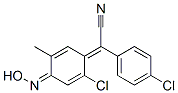
- Chemical Name:(4-chlorophenyl)[2-chloro-4-(hydroxyimino)-5-methylcyclohexa-2,5-dien-1-ylidene]acetonitrile
- CAS:844-24-6
- MF:C15H10Cl2N2O
- Structure:
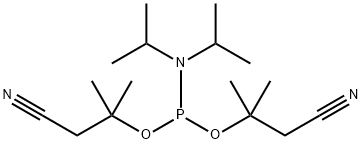
- Chemical Name:Bis(2-cyano-1,1-dimethylethyl) N,N-bis(1-methylethyl)phosphoramidite
- CAS:916996-01-5
- MF:C16H30N3O2P
- Structure:
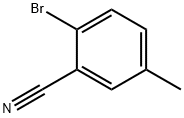
- Chemical Name:2-Bromo-5-methylbenzonitrile
- CAS:42872-83-3
- MF:C8H6BrN
- Structure:
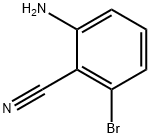
- Chemical Name:2-amino-6-bromobenzonitrile
- CAS:77326-62-6
- MF:C7H5BrN2
- Structure:
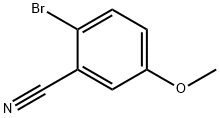
- Chemical Name:2-Bromo-5-methoxybenzonitrile
- CAS:138642-47-4
- MF:C8H6BrNO
- Structure:
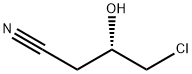
- Chemical Name:(S)-4-Chloro-3-hydroxybutyronitrile
- CAS:127913-44-4
- MF:C4H6ClNO
- Structure:
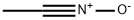
- Chemical Name:1-Aza-1-propyne1-oxide
- CAS:7063-95-8
- MF:C2H3NO
- Structure:
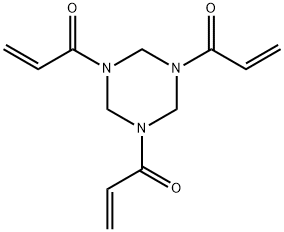
- Chemical Name:1,3,5-Triacryloylhexahydro-1,3,5-triazine
- CAS:959-52-4
- MF:C12H15N3O3
- Structure:
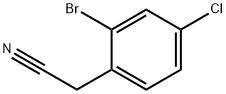
- Chemical Name:2-BROMO-4-CHLOROPHENYLACETONITRILE
- CAS:52864-54-7
- MF:C8H5BrClN
- Structure:
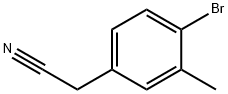
- Chemical Name:2-(4-bromo-3-methylphenyl)acetonitrile
- CAS:215800-25-2
- MF:C9H8BrN
- Structure:
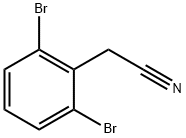
- Chemical Name:2-(2,6-dibroMophenyl)acetonitrile
- CAS:67197-53-9
- MF:C8H5Br2N
- Structure:
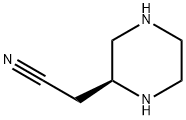
- Chemical Name:(S)-2-(piperazin-2-yl)acetonitrile
- CAS:1589082-26-7
- MF:C6H11N3
- Structure:

- Chemical Name:5-Bromo-3-fluoro-pyridine-2-carbonitrile
- CAS:886373-28-0
- MF:C6H2BrFN2
- Structure:
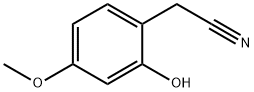
- Chemical Name:Benzeneacetonitrile, 2-hydroxy-4-methoxy-
- CAS:32884-26-7
- MF:C9H9NO2
- Structure:
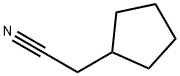
- Chemical Name:cyclopentaneacetonitrile
- CAS:5732-87-6
- MF:C7H11N
- Structure:
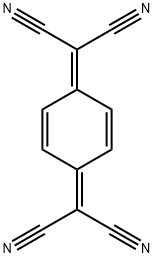
- Chemical Name:7,7,8,8-Tetracyanoquinodimethane
- CAS:1518-16-7
- MF:C12H4N4
- Structure:
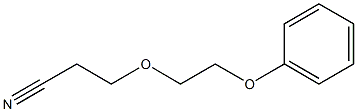
- Chemical Name:Propanenitrile,3-(2-phenoxyethoxy)-
- CAS:6328-54-7
- MF:C11H13NO2
- Structure:
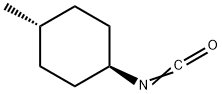
- Chemical Name:trans-4-Methycyclohexyl isocyanate
- CAS:32175-00-1
- MF:C8H13NO
- Structure:
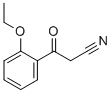
- Chemical Name:2-ETHOXYBENZOYLACETONITRILE
- CAS:89-44-1
- MF:C11H11NO2
- Structure:
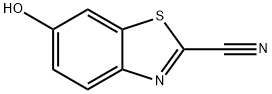
- Chemical Name:2-Cyano-6-hydroxybenzothiazole
- CAS:939-69-5
- MF:C8H4N2OS
- Structure:
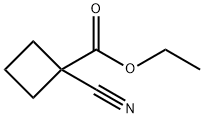
- Chemical Name:1-CYANOCYCLOBUTANECARBOXYLIC ACID ETHYL ESTER
- CAS:28246-87-9
- MF:C8H11NO2
- Structure:
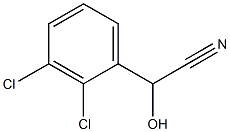
- Chemical Name:(2,3-dichloro)hydroxyacetonitrile
- CAS:93842-97-8
- MF:C8H5Cl2NO
- Structure:
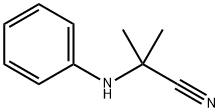
- Chemical Name:2-anilino-2-methylpropiononitrile
- CAS:2182-38-9
- MF:C10H12N2
- Structure:
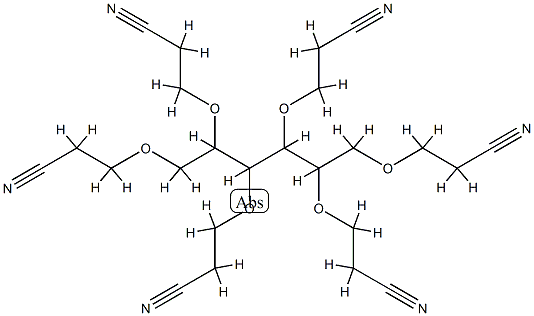
- Chemical Name:1,2,3,4,5,6-hexakis-O-(2-cyanoethyl)hexitol
- CAS:2465-92-1
- MF:C24H32N6O6
- Structure:
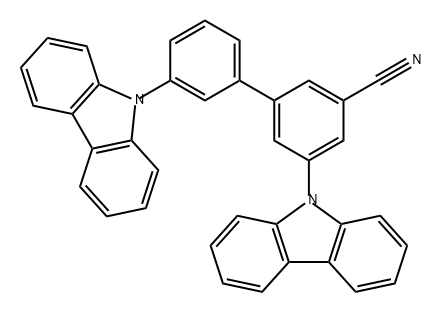
- Chemical Name:mCBP-CN
- CAS:1327163-09-6
- MF:C37H23N3
- Structure:
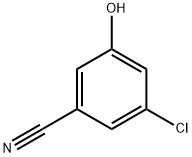
- Chemical Name:3-chloro-5-hydroxy-benzonitrile
- CAS:473923-97-6
- MF:C7H4ClNO
- Structure:
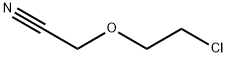
- Chemical Name:2-chloroethoxyacetonitrile
- CAS:31250-08-5
- MF:C4H6ClNO
- Structure:
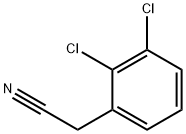
- Chemical Name:2,3-Dichlorophenylacetonitrile
- CAS:3218-45-9
- MF:C8H5Cl2N
- Structure:
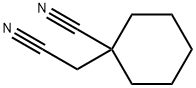
- Chemical Name:1-CYANOCYCLOHEXANE ACETONITRILE
- CAS:4172-99-0
- MF:C9H12N2
- Structure:
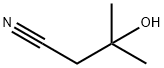
- Chemical Name:3-HYDROXY-3-METHYLBUTYRONITRILE
- CAS:13635-04-6
- MF:C5H9NO
- Structure:
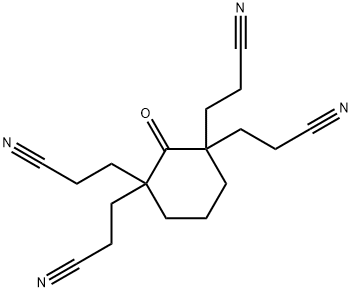
- Chemical Name:3-[1,3,3-tris(2-cyanoethyl)-2-oxo-cyclohexyl]propanenitrile
- CAS:5883-05-6
- MF:C18H22N4O
- Structure:
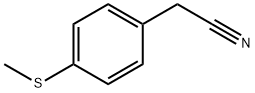
- Chemical Name:P-(METHYLTHIO)PHENYLACETONITRILE
- CAS:38746-92-8
- MF:C9H9NS
- Structure:
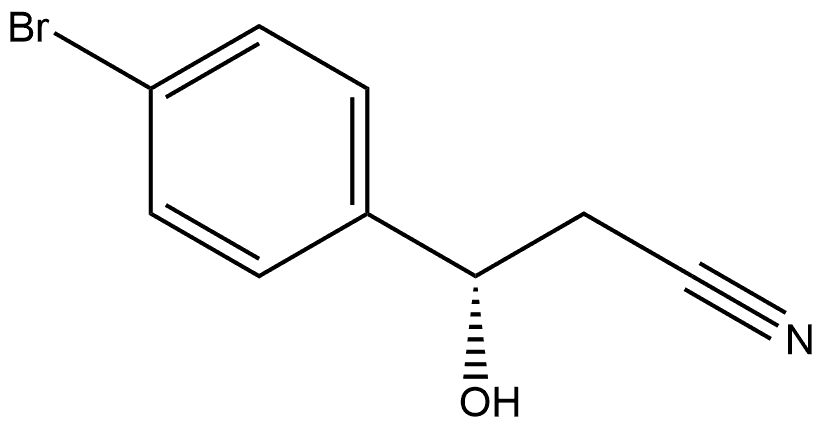
- Chemical Name:(S)-3-(4'-bromophenyl)-3-hydroxypropanenitrile
- CAS:877876-70-5
- MF:C9H8BrNO
- Structure:
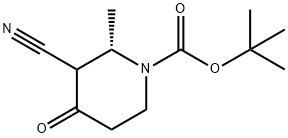
- Chemical Name:(2S)-tert-Butyl 3-cyano-2-methyl-4-oxopiperidine-1-carboxylate
- CAS:2212021-56-0
- MF:C12H18N2O3
- Structure:
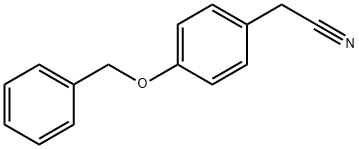
- Chemical Name:4-Benzyloxyphenylacetonitrile
- CAS:838-96-0
- MF:C15H13NO