N-Boc-3-哌啶甲酸乙酯的合成及其应用
发布日期:2022/11/1 13:55:18
简介
N-Boc-3-哌啶甲酸乙酯的CAS号是130250-54-3,分子式是C13H23NO4,分子量是257.33,熔点是31-35℃,沸点是95℃/0.5mm,密度是1.077±0.06g/cm3(Predicted),以及酸度系数(pKa)是-2.40±0.40(Predicted)。N-Boc-3-哌啶甲酸乙酯的最常见用途是作为食品添加剂。还可用作有机合成原料,纸张处理剂,纤维工业的柔软剂,动物胶的软化剂,还用作测定大米中氨基酸含量的分析试剂[1]。在有机合成中,N-Boc-3-哌啶甲酸乙酯在医药方面的用途居多,在农药、染料,颜料,香料,助剂方面也有很多用途[2-3]。
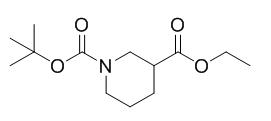
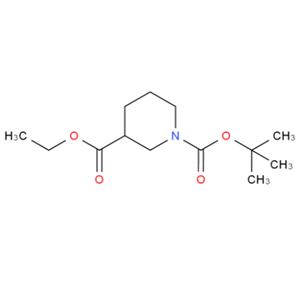
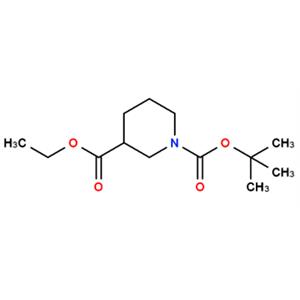
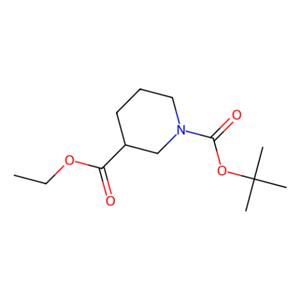
图1 N-Boc-3-哌啶甲酸乙酯的结构式。
合成
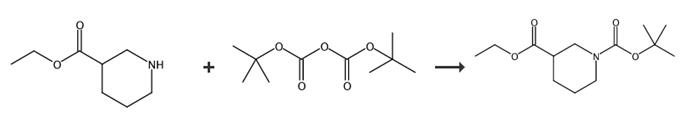
图2 N-Boc-3-哌啶甲酸乙酯的合成路线[4-7]。
方法一:在室温下向存于THF(159 mL)中的哌啶-3-羧酸乙酯(15 g,95 mmol)溶液中添加Boc2O(7.75 mL,33.4 mmol),然后在室温下搅拌16 h。用水(50 mL)稀释所得反应质量,然后用乙酸乙酯(3x100 mL)萃取。用饱和NaHCO3溶液(1 x200 mL)、盐水(20 mL)洗涤组合有机提取物,干燥(Na2SO4),过滤并浓缩。所得残留物通过ELSD ISCO在120 g硅胶柱上纯化,在己烷中洗脱约25%乙酸乙酯的化合物,收集并浓缩纯馏分,得到所需产物1-叔丁基3-乙基哌啶-1,3-二羧酸盐。得到最终产物N-Boc-3-哌啶甲酸乙酯,收率23.39 g,91 mmol,95%。对于C13H23NO4 m/z 257.3,发现:158.2(m-Boc+H)+;1H核磁共振(400 MHz,CDCl3)δppm 4.13(q,J 7.3Hz,2H),3.90(d,J 13.1 Hz,1H),2.87-3.08(m,1H)。合成路线如图2所示。
方法二:将尼泊金乙酯(1.5 g,0.0095 mol)在0-5°C下逐滴添加到二叔丁基二碳酸酯(2.17 g,0.0099 mol)和三乙胺(1.4 mL,0.0099 mo)存于二氯甲烷(25 mL)中的溶液中。添加催化量的二甲氨基吡啶,并将混合物在0-5°C下搅拌15分钟。将溶液加热至室温并搅拌18小时。浓缩反应混合物。将油在真空下干燥2小时。材料使用时未经净化。得到最终产物N-Boc-3-哌啶甲酸乙酯,MS(ESI)m/z 296[m+K]+。合成路线如图2所示。
方法三:将二碳酸二叔丁酯(11.46 g,52.5 mmol)添加到二氯甲烷(120 mL)中的尼泊金乙酯(7.86 g,50 mmol)的冷(0℃)溶液中。在室温下将所得混合物搅拌4小时。用5%碳酸氢钠水溶液稀释反应混合物。分离有机相,经硫酸镁干燥,过滤并减压蒸发。获得最终产物N-Boc-3-哌啶甲酸乙酯(12.8 g,收率;100%)。合成路线如图2所示。
方法四:将1-叔丁基3-乙基哌啶-1,3-二羧酸乙酯-尼泊金乙酯(1.55 mL,10 mmol)、(Boc)2O(2.4 g,1.1 equiv)、三乙胺(2.8 mL,2.1 equiv”)和二氯甲烷(70 mL)混合并在室温下搅拌过夜。用EtOAc(200 mL)稀释反应混合物,用5%aq HCl(2 x 25 mL)洗涤,放置aq NaHCO3(30 mL)和盐水(20 mL),并在Na2SO4上干燥。浓缩后,粗产物在40g硅胶柱上通过色谱纯化,用0-30%EtOAc在己烷梯度中洗脱,得到最终产物N-Boc-3-哌啶甲酸乙酯(2.61g,100%)。合成路线如图2所示。
应用
N-Boc-3-哌啶甲酸乙酯及其衍生物是重要的药物中间体,具有很高的生物活性[8]。N-Boc-3-哌啶甲酸乙酯可用于合成消炎药物类胰蛋白酶抑制剂,起到在受体水平上拮抗炎症介质的作用。N-Boc-3-哌啶甲酸乙酯还可用于合成抗肿瘤药基质金属蛋白酶抑制剂和法尼基转移酶抑制剂[9]。总之,N-Boc-3-哌啶甲酸乙酯及其衍生物具有良好的生物活性,不仅可用于合成GABA 摄入抑制剂、抗肿瘤药,还可以合成生长激素促分泌素、消炎镇痛药物、心血管药物、促智药物、抗流感病毒药物和骨疾病药物等[10-12]。
风险
N-Boc-3-哌啶甲酸乙酯是有毒性的,短时间暴露在喹啉蒸汽中会导致鼻子、眼睛和呼吸道被腐蚀,也可能导致头昏和恶心[13]。长时间暴露的影响还不确定,不过喹啉与肝损伤有一定的关系。
参考文献
[1] S. Qi, Q. Peng, D. Xu, C. Guo, J. Yang, H. Dong, Y. Wang, F. Wu, S. Chen, In situ structural evolutions of sulfur-limonene polysulfides encapsulated in yeast-derived porous N-doped carbon spheres for high-performance Li-S batteries, Mater. Today Chem. (2022) Ahead of Print.
[2] G. Digilio, S. Lacerda, B. Lavin Plaza, A. Phinikaridou, Extracellular Matrix Targeted MRI Probes, Analysis Sensing (2022) Ahead of Print.
[3] J.S. Samuelian, D.A. Baum, Identification of DNA Aptamers for Benzodiazepine Detection in Synthetic Urine, Analysis Sensing (2022) Ahead of Print.
[4] S. Fortunati, F. Pedrini, E. Del Grosso, L. Baranda Pellejero, A. Bertucci, Design of Specific Nucleic Acid-Based Biosensors for Protein Binding Activity, Analysis Sensing (2022) Ahead of Print.
[5] D. Cavazos-Elizondo, A. Aguirre-Soto, Photophysical Properties of Fluorescent Labels: A Meta-Analysis to Guide Probe Selection Amidst Challenges with Available Data, Analysis Sensing (2022) Ahead of Print.
[6] D. Chen, Y. Wu, Rapid and Ultrasensitive Electrochemical Detection of TP53 Gene Mutation in Blood: Hybridization with a DNA/Gold-Coated Magnetic Nanoparticle Network, Analysis Sensing (2022) Ahead of Print.
[7] L. Liu, J. Guo, X. Liu, A. Wang, X. Yu, L. Ding, Photoelectrochemical Clothianidin Detection Based on a WO3/CdS Heterostructure Coated with a Molecularly Imprinted Thin Film, Analysis Sensing (2022) Ahead of Print.
[8] A.M. Yashchenok, V.S. Chernyshev, E.V. Konovalova, R. Kholodenko, E. Tsydenzhapova, V.O. Shipunova, A.A. Schulga, S.M. Deyev, D.A. Gorin, Anti-CD63-Oligonucleotide Functionalized Magnetic Beads for the Rapid Isolation of Small Extracellular Vesicles and Detection of EpCAM and HER2 Membrane Receptors using DARPin Probes, Analysis Sensing (2022) Ahead of Print.
[9] E.E. Hickey, A. Shigemoto, A. Gericke, S.C. Burdette, Isothermal Titration Calorimetry for Characterizing the Zinc(II)-Binding Properties of Photocaged Complexes with Micromolar Affinity, Analysis Sensing (2022) Ahead of Print.
[10] K.H. Jensen, B.W. Michel, Detection of Ethylene with Defined Metal Complexes: Strategies and Recent Advances, Analysis Sensing (2022) Ahead of Print.
[11] H. Zhao, F. Yu, M. Yu, L. Li, DNA-Based Biosensors for Imaging of Subcellular Enzymatic Activity, Analysis Sensing (2022) Ahead of Print.
[12] T. Waldmann, R.-G. Scurtu, D. Brandle, M. Wohlfahrt-Mehrens, Effects of Tab Design in 21700 Li-Ion Cells: Improvements of Cell Impedance, Rate Capability, and Cycling Aging, Energy Technol. (Weinheim, Ger.) (2022) Ahead of Print.
[13] F. Schomburg, R. Drees, M. Kurrat, M.A. Danzer, F. Roder, Characterization of the Solid-Electrolyte Interphase Growth During Cell Formation Based on Differential Voltage Analysis, Energy Technol. (Weinheim, Ger.) (2022) Ahead of Print.
欢迎您浏览更多关于N-Boc-3-哌啶甲酸乙酯的相关新闻资讯信息