BOC-N-甲基-L-丙氨酸的合成及其应用
发布日期:2022/9/6 14:00:32
基本描述
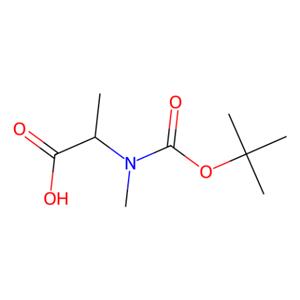
BOC-N-甲基-L-丙氨酸,英文名为boc-N-methyl-L-alanine,CAS号为16948-16-6,分子式是C9H17NO4,分子量是203.2356,其是常用的化工中间体一种。另外,BOC-N-甲基-L-丙氨酸不仅可用于蛋白质的合成,在食品领域还可以用作增味剂、防腐剂、保鲜剂,在医药领域可用于药物原料,在日化领域可用于温和表面活性剂合成[1]。目前,BOC-N-甲基-L-丙氨酸的生产方法主要有化学合成法、水解提取法、酶转化法以及微生物发酵法。化学合成法生产的BOC-N-甲基-L-丙氨酸质量较差,生产过程易造成环境污染;水解提取法生产过程较复杂,不适宜规模化和工业化生产[2]。
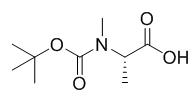
图1 BOC-N-甲基-L-丙氨酸的结构式。
合成
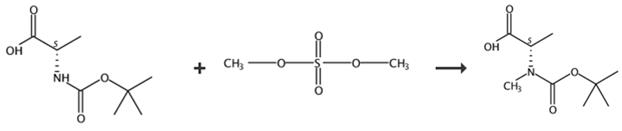
图2 BOC-N-甲基-L-丙氨酸的合成路线[3。
加入l - boc -丙氨酸(5 g, 26.4 mmol)的四氢呋喃(80 mL)溶液,在0℃下,分次加入细粉KOH (10.4 g, 187 mmol),然后加入四丁基硫酸氢铵(0.5 g,重量10%)。然后,滴加硫酸二甲酯(10 mL, 105 mmol)超过15分钟。再搅拌30分钟,加入水(50 mL)。室温搅拌5h后,加入20%氢氧化铵水溶液(20 mL)。用乙醚(100 mL)稀释反应,分离水层,用饱和NaHCO3水溶液(2 × 40 mL)提取有机层。混合水层用1M KHSO4酸化至pH为1,用乙酸乙酯(2x200 mL)提取。有机层经过组合、干燥(Na2SO4)、过滤和浓缩。生成的产物鉴定为BOC-N-甲基-L-丙氨酸。黄油,产量4.3 g, 80%。1 h - nmr (500 MHz, CDCl3): 1.43 - -1.55 (m, 12 h), 2.91 (br年代,3 h), 4.54 - -4.58 (m, 1 h), 4.89-4.93 (m, 1 h)。
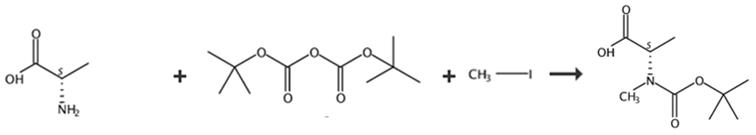
图3 BOC-N-甲基-L-丙氨酸的合成路线[4]。
将(L)-丙氨酸(4g)和碳酸钠(9.5g,90mmol,2当量)溶解在H2O/THF混合物(60ml,1:1)中。将反应混合物冷却至0℃。向反应混合物中加入Boc2O(10.8g)。让反应升温至室温。搅拌反应混合物24小时。通过小心加入100ml 1M HCl酸化反应混合物,直至达到pH=2。
用5×80ml EtOAc萃取反应混合物。用盐水洗涤合并的有机层。在无水硫酸镁上干燥合并的有机层。蒸发合并的有机层得到产品BOC-N-甲基-L-丙氨酸。(400 MHz, DMSO-d6) Mixture of rotamers: δ 1.21 (d, J = 7.3 Hz, 3H, H3), 1.30-1.44 (m, 9H, tBu), 3.77-3.97(m, 1H, H2), 6.66-6.74 (m, 1H, NH), 7.06 (d, J = 7.5 Hz, 1H, NH), 12.37 (s, 1H, COOH). (101 MHz, DMSO-d6) δ 17.1 (q, C3), 28.2 (q, tBu), 48.8 (d, C2), 77.9 (s, tBu), 155.3 (s, N-CO-O), 174.7 (COOH).
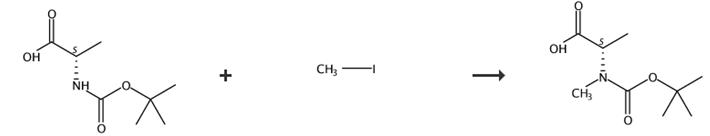
图4 BOC-N-甲基-L-丙氨酸的合成路线[5]。
在氩气下将(L)-N-Boc-丙氨酸(6g)溶于无水THF(100ml)中。将反应混合物冷却至0℃。将氢化钠(60%在矿物油中,3.8克,95.2毫摩尔,3当量)分三份加入混合物中。将反应混合物在0℃搅拌1小时。通过注射器向混合物中加入甲基碘(16 mL,254 mmol)。让反应升温至室温。将反应混合物连续搅拌16小时。用300ml水淬灭反应。用100ml Et2O萃取混合物。分离有机层。用30ml饱和NaHCO3萃取有机层。用6M HCl酸化合并的水层至pH=3。用3×100毫升EtOAc萃取合并的水层。用2×50毫升饱和Na2S2O3洗涤合并的有机层。在无水硫酸钠上干燥合并的有机层。蒸发合并的有机层得到白色固体产品BOC-N-甲基-L-丙氨酸。核磁数据如下:(400 MHz, DMSO-d6) Mixture of rotamers: δ 1.24-1.33 (m, 3H, H3), 1.32-1.45 (m, 9H, tBu),2.73 (s, 3H, NCH3), 4.17-4.39 (m, 1H, H2), 4.44-4.68 (m, 1H, H2). (101 MHz, DMSO-d6) Mixture of rotamers: δ 14.6 (q, C3), 15.2 (q, C3), 27.9 (q, tBu), 28.0 (q, tBu), 30.6 (q, NMe), 31.5 (q, NMe), 53.2 (d, CH), 54.8 (d, CH), 79.0 (s, tBu), 154.6 (s, N-CO-O), 155.0 (s, N-CO-O), 173.3 (s, CO), 173.4 (s, CO).
应用
BOC-N-甲基-L-丙氨酸可用于合成绿色环保的温和氨基酸表面活性剂的中间体。相较于传统的有机化学表面活性剂,其合成的表面活性剂不仅能够达到相同的效果,而且去污、乳化和渗透能力强,不会污染环境,还具有优良的爽肤和保湿能力,对人体健康,可适用于婴幼儿和敏感性的皮肤[6-9]。BOC-N-甲基-L-丙氨酸可用于生产新型螯合剂甲基甘氨酸二乙酸(methyl glycine diacetic acid,MGDA),MGDA可替代新型高性能环保洗涤剂中的含磷螯合剂,可以在水中自然降解,避免了传统磷酸盐对环境的负面影响,避免对人体和环境造成危害[10-11]。BOC-N-甲基-L-丙氨酸也可用于合成聚酯酰胺,这是一种新的可生物降解的聚合物,可用于生产具有定制性能的各种材料[12]。BOC-N-甲基-L-丙氨酸还可作为催化剂催化羟醛缩合反应,催化剂可重复利用5次以上。
参考文献
[1] M. Asano, K. Hashimoto, B. Saito, Z. Shiokawa, H. Sumi, M. Yabuki, M. Yoshimatsu, K. Aoyama, T. Hamada, N. Morishita, D.R. Dougan, C.D. Mol, S. Yoshida, T. Ishikawa, Design, stereoselective synthesis, and biological evaluation of novel tri-cyclic compounds as inhibitor of apoptosis proteins (IAP) antagonists, Bioorg. Med. Chem. 21(18) (2013) 5725-5737.
[2] A. Flohr, G. Galley, R. Norcross, N. Zorn, Preparation of dimeric SMAC peptidomimetics as BIR2 and BIR3 domains of IAP protein inhibitors for use in treating cancer, F. Hoffmann-La Roche AG, Switz.; Hoffmann-La Roche Inc. . 2017, p. 148pp.
[3] B. Hinzen, H. Brotz, R. Endermann, K. Henninger, H. Paulsen, S. Raddatz, T. Lampe, V. Hellwig, A. Schumacher, Synthesis of antibacterial macrocyclic oligopeptides for use in treatment of diseases in humans or animals, Bayer Aktiengesellschaft, Germany; Bayer Healthcare AG . 2003, p. 140 pp.
[4] Y. Kawazoe, Y. Tanaka, S. Omura, D. Uemura, Design, synthesis, and evaluation of derivatives of the fat-accumulation inhibitor ternatin: toward ternatin molecular probes, Tetrahedron Lett. 55(32) (2014) 4445-4447.
[5] D.K. Koelmel, R.P. Loach, T. Knauber, M.E. Flanagan, Employing Photoredox Catalysis for DNA-Encoded Chemistry: Decarboxylative Alkylation of α-Amino Acids, ChemMedChem 13(20) (2018) 2159-2165.
[6] A.H. Miah, I.E.D. Smith, M. Rackham, A. Mares, A.R. Thawani, R. Nagilla, P.A. Haile, B.J. Votta, L.J. Gordon, G. Watt, J. Denyer, D.T. Fisher, P. Dace, P. Giffen, A. Goncalves, I. Churcher, P. Scott-Stevens, J.D. Harling, Optimization of a Series of RIPK2 PROTACs, J. Med. Chem. 64(17) (2021) 12978-13003.
[7] M. Mitomi, M. Sakai, R. Horikoshi, Y. Onozaki, S. Nakamura, S. Omura, T. Sunazuka, T. Hirose, K. Shiomi, R. Masuma, Preparation of cyclic depsipeptide as pest control agent, Meiji Seika Pharma Co., Ltd., Japan; The Kitasato Institute . 2013, p. 127pp.
[8] Y. Nagato, S. Mizumoto, T. Murakami, T. Tanaka, Preparation of nitrogen-containing heterocyclic compound and synthetic intermediate thereof, Fujifilm Corp., Japan . 2017, p. 38pp.; Chemical Indexing Equivalent to 166:156030 (WO).
[9] Y. Nagato, S. Mizumoto, T. Murakami, T. Tanaka, Preparation of nitrogen-containing heterocyclic compound and synthetic intermediate thereof, Fujifilm Corporation, Japan . 2017, p. 59pp.; Chemical Indexing Equivalent to 166:165918 (JP).
[10] C. Pissot Soldermann, J. Quancard, A. Schlapbach, O. Simic, M. Tintelnot-Blomley, T. Zoller, Preparation of pyrazolopyrimidine derivatives and their use as MALT1 inhibitors, Novartis AG, Switz. . 2015, p. 161pp.
[11] F. Sun, C.Z. Ding, Z. Cai, W. Qian, G. Hu, J. Li, S. Chen, Crystal form of IAP antagonist and preparation method therefor, Medshine Discovery Inc., Peop. Rep. China . 2019, p. 28pp.
[12] F. Tripodi, F. Dapiaggi, F. Orsini, R. Pagliarin, G. Sello, P. Coccetti, Synthesis and biological evaluation of new 3-amino-2-azetidinone derivatives as anti-colorectal cancer agents, MedChemComm 9(5) (2018) 843-852.
[13] D.C.J. Waalboer, J.A. Muns, N.J. Sijbrandi, R.B.M. Schasfoort, R. Haselberg, G.W. Somsen, H.-J. Houthoff, G.A.M.S. van Dongen, Platinum(II) as bifunctional linker in antibody-drug conjugate formation: Coupling of a 4-nitrobenzo-2-oxa-1,3-diazole fluorophore to trastuzumab as a model, ChemMedChem 10(5) (2015) 797-803.
欢迎您浏览更多关于BOC-N-甲基-L-丙氨酸的相关新闻资讯信息