-
물성
물에는 거의 녹지 않으나, 에탄올·에테르·클로로폼 등의 유기용매에는 잘 녹는다.
-
용도
산화를 방지하며 트고 거칠어진 피부를 개선하는데 도움을 주며 피부에 보습기능을 향상시켜주고
자극 받은 피부를 진정, 보호해 피부장벽을 강화 시켜주는데 도움을 줍니다.
-
용도
예전에는 힙논이라는 이름의 최면제로서 사용되었으나, 현재는 향료와 수지 등을 제조할 때에 쓰이는 원료이다.
-
순도시험
(1) 응고점 : 이 품목의 응고점은 19℃이상이어야 한다.
(2) 굴절률 : 이 품목의 굴절률 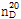 은 1.533~1.535이어야 한다.
은 1.533~1.535이어야 한다.
(3) 용상 : 이 품목 1mL를 50% 에탄올 5mL에 녹일 때, 그 액은 징명하여야 한다.
(4) 염소화합물 : 이 품목은 향료시험법 중 할로겐시험법의 동망법에 따라 시험할 때, 이에 적합하여야 한다.
-
확인시험
(1) 이 품목 1방울에 물 1mL를 가하여 잘 섞고 니트로프루시드나트륨시액 2방울을 넣은 후 수산화나트륨용액(3→10) 2방울을 넣어 흔들어 섞으면 암적색을 나타낸다. 이에 다시 초산 5방울을 넣으면 진한 청색을 나타낸다.
(2) 이 품목 1g에 세미카르바지드염산염 5g 및 초산칼륨 5g을 물 15mL에 녹인 액 5mL를 가한 후 에탄올 5mL를 가하여 잘 흔들어 섞어 방치하면 백색결정이 생긴다. 이 결정을 취해 묽은에탄올을 용매로 하여 재결정할 때, 그 융점은 약 198℃이다.
-
정량법
이 품목 약 1g을 정밀히 달아 향료시험법 중 알데히드류 및 케톤류함량측정법의 히드록실아민법 중 제2법에 따라 시험한다. 단, 가열시간은 1시간으로 한다.
0.5N 염산 1mL = 60.08mg C8H8O
-
개요
Acetophenone is the simplest aromatic ketone and is a clear liquid/crystal and very slightly
soluble in water with a sweet pungent taste and odour resembling oranges. It is used as
a polymerisation catalyst for the manufacture of olefins. Acetophenone is used in perfumery
as a fragrance ingredient in soaps, detergents, creams, lotions, and perfumes; as a
flavouring agent in foods, non-alcoholic beverages, and tobacco; as a specialty solvent for plastics and resins; as a catalyst for the polymerisation of olefins; and as a photosensitiser
in organic syntheses. Acetophenone is a raw material for the synthesis of some pharmaceuticals
and is also listed as an approved excipient by the U.S. FDA. Acetophenone occurs
naturally in many foods such as apple, apricot, banana, and beef. Acetophenone has been detected in ambient
air and drinking water; exposure of the general public may occur through the inhalation
of contaminated air or the consumption of contaminated water. It is highly flammable
and will get easily ignited by heat, sparks, or flames, and the vapours may form explosive
mixtures with air.
-
화학적 성질
Acetophenone is a colorless, oily liquid with a sweet, floral odor.It is a naturally occurring component of a large number of foods and essential oils.
Acetophenone can be hydrogenated catalytically to 1-phenylethanol. It is obtained as a by-product in the Hock phenol synthesis and is purified from the high-boiling residue by distillation. The quantities obtained from this source satisfy the present demand.
Acetophenone is used for perfuming detergents and industrial products and is an intermediate in the synthesis of other fragrance materials.
-
출처
Reported found in cocoa, beef, raspberry, peas, and concord grape
-
용도
Acetophenone is a reagent used in the production of fragrances and resin polymers.
-
정의
ChEBI: Acetophenone is a methyl ketone that is acetone in which one of the methyl groups has been replaced by a phenyl group. It has a role as a photosensitizing agent, an animal metabolite and a xenobiotic.
-
생산 방법
Most methyl phenyl ketone originates from the Hock process for the production of phenol from isopropylbenzene (→Phenol); it is isolated from the residue of this process. In addition, acetophenone can be obtained as a main product by selective decomposition of cumene hydroperoxide in the presence of copper catalysts at 100℃:
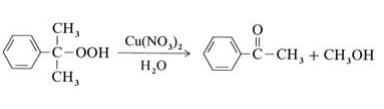
A second possibility is the oxidation of ethylbenzene with air or oxygen at 130℃ and 0.5 MPa. Catalysts used include cobalt salts or manganese salts of naphthenic or fatty acids. Conversion of ethylbenzene is limited to ca. 25 % to minimize the byproducts 1-phenylethanol and benzoic acid. A third method is the Friedel – Crafts acetylation of benzene with acetic anhydride, but this is not of industrial importance.
-
제조 방법
From benzene and acetylchloride in the presence of aluminum chloride or by catalytic oxidation of ethyl benzene; also
prepared by fractional distillation and crystallization from the essential oil of Stirlingia latifolia.
-
일반 설명
Acetophenone appears as a colorless liquid with a sweet pungent taste and odor resembling the odor of oranges. Freezes under cool conditions. Slightly soluble in water and denser than water. Hence sinks in water. Vapor heavier than air. A mild irritant to skin and eyes. Vapors can be narcotic in high concentrations. Used as a flavoring, solvent, and polymerization catalyst.
-
공기와 물의 반응
Slightly soluble in water.
-
반응 프로필
Acetophenone reacts with many acids and bases liberating heat and flammable gases (e.g., H2). Reacts with many oxidizing agents. Reacts with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. The amount of heat in these reactions may be sufficient to start a fire in the unreacted portion. Incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides.
-
건강위험
Acetophenone is an irritant, mutagen, and amildly toxic compound. In rabbits 0.77 mgproduced severe eye irritation, but the actionon skin was mild. In mice, subcutaneousadministration of this compound producedsleep; a dose of 330 mg/kg was lethal.
LD50 value, intraperitoneal (mice): 200mg/kg
No symptoms of severe toxicity, nor its carcinogenicityin humans, has been reported..
-
화재위험
Combustible liquid; flash point (closed cup)
82°C (180°F); vapor pressure 1 torr at
37°C (98.6°F); vapor density 4.1 (air = 1);
autoignition temperature 570°C (1058°F);
fire-extinguishing agent: dry chemical, foam,
or CO2; water may cause frothing, but it can
be used to flush and dilute the spill. Its reaction
with strong oxidizers may be violent.
-
Safety Profile
Poison by intraperitoneal and subcutaneous routesModerately toxic by ingestion. A skin and severe eye irritant. Mutation data reported. Narcotic in high concentration. A hypnotic. Flammable liquid. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and fumes. See also IGTONES
-
잠재적 노출
Acetophenone is used as a solvent and in perfume manufacture to impact a pleasant jasmine or orange-blossom odor. It is used as a catalyst in olefin polymerization and as a flavorant in tobacco. It is also used in the synthesis of pharmaceuticals
-
Carcinogenicity
No carcinogenicity studies were
identified for acetophenone. The U.S. EPA has classified
acetophenone as a Category D, not classifiable as to human
carcinogenicity.
-
환경귀착
It is unclear what mechanism is responsible for the central
nervous system depression observed following high doses of
acetophenone. In vitro evaluations have demonstrated that
acetophenone suppresses voltage-gated ion channels in olfactory
receptor cells and retinal neurons; however, it is unclear if
this is related to any of the observed toxicity in animal studies.
-
신진 대사
At one time, acetophenone was used as a hypnotic. Its conversion to benzoic acid and methylphenylcarbinol in dogs and rabbits was observed by a number of early workers. Small amounts are also excreted as mandelic acid. In the rabbit about half the dose is excreted as methylphenylcarbinyl glucuronide and about 20 % as hippuric acid. It is probable that the ketone is first asymmetrically reduced to the carbinol, which is the precursor of benzoic and mandelic acids.
-
운송 방법
UN1993 Flammable liquids, n.o.s., Hazard Class: 3; Labels: 3-Flammable liquid, Technical Name Required.
-
Purification Methods
Dry it by fractional distillation or by standing with anhydrous CaSO4 or CaCl2 for several days, followed by fractional distillation under reduced pressure (from P2O5, optional), and careful, slow and repeated partial crystallisations from the liquid at 0o excluding light and moisture. It can also be crystallised at low temperatures from isopentane. Distillation can be followed by purification using gas-liquid chromatography [Earls & Jones J Chem Soc, Faraday Trans 1 71 2186 1975.] [Beilstein 7 H 271, 7 IV 619.] § A commercial polystyrene supported version is available — scavenger resin (for diol substrates).
-
비 호환성
May form explosive mixture with air. See flash point, above. Reacts violently with strong oxidizers, many acids, bases, amines, amides, and inorganic hydroxides; alkali metals; hydrides, and nitrides. Reacts with reducing agents; alkali metals; hydrides, nitrides. Contact with all preceding materials release heat and flammable gases, including hydrogen; the heat may be sufficient enough to result in fire. Incompatible with aldehydes, aliphatic amines, alkanolamines, cyanides, isocyanates, organic acids, peroxides; perchloric acid. May attack plastics, and some rubbers and coatings
-
폐기물 처리
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration, preferably with a flammable solvent

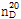 은 1.533~1.535이어야 한다.
은 1.533~1.535이어야 한다.