-
外観
無色~わずかにうすい黄色, 澄明の液体
-
性質
臭化エチルの融点は−119°Cで、沸点は38.4°Cです。常温では無色の液体で、揮発性があり、エーテルのような臭いがあります。蒸気は空気より重く、引火点は-2°C以下で、引火しやすいです。爆発範囲は6.8~8.0%です。
水に溶けにくく、20°Cで水100gに0.914g溶解します。エタノールやベンゼンのような有機溶媒には溶けます。
一般的なハロゲン系炭化水素と同様に、健康に悪影響があり、経口・吸入ともに許容濃度は200ppmです。吸入した場合には、鼻やのどを強く刺激して、頭痛、動悸、瞳孔拡大、視力障害、顔面紅潮などを起こし、チアノーゼや呼吸困難などが起きる可能性もあります。皮膚からも吸収されて同様の中毒症状を起こし、眼に入ると眼の粘膜が刺激され炎症が起きます。
-
溶解性
水に難溶 (0.914g/100ml), アルコール, エーテル等各種有機溶剤にはよく溶ける。
-
解説
C2H5Br(108.97).ハロゲン化アルキルの一つ。ブロモエタンは,エーテル臭の揮発性液体.引火点-20 ℃,融点-125.5 ℃,沸点38.4 ℃.d2041.4555.n20D1.4238.臭化エチル、エチルブロミドともよぶ。エタンの水素原子1個を臭素原子で置換したもの。無色の液体。水に難溶、エタノール(エチルアルコール)、ベンゼンなどの有機溶媒に可溶。経口・吸入ともに有毒(許容濃度200ppm)。エタノールに臭化水素酸と少量の硫酸を加えて蒸留すれば得られる。

臭化エチルともいう.エタノールに臭化水素と硫酸とを加えて蒸留すると得られる.森北出版「化学辞典(第2版)
-
用途
ブロモエタンは,有機合成でエチル化剤として利用される。また、麻酔剤としても用いられる。
-
用途
有機合成原料、冷凍剤
-
用途
真用、I R 用測定粉末
-
用途
有機合成原料、医薬品、冷凍剤原料、農薬原料
-
構造
臭化エチルはエタンが有する1個の水素原子を、臭素原子で置換した化合物です。示性式はCH3CH2Brと表されます。EtBrと略される場合もあります。分子量は109.0g/molで、密度は1.4g/cm3です。
-
合成
臭化エチルの合成法
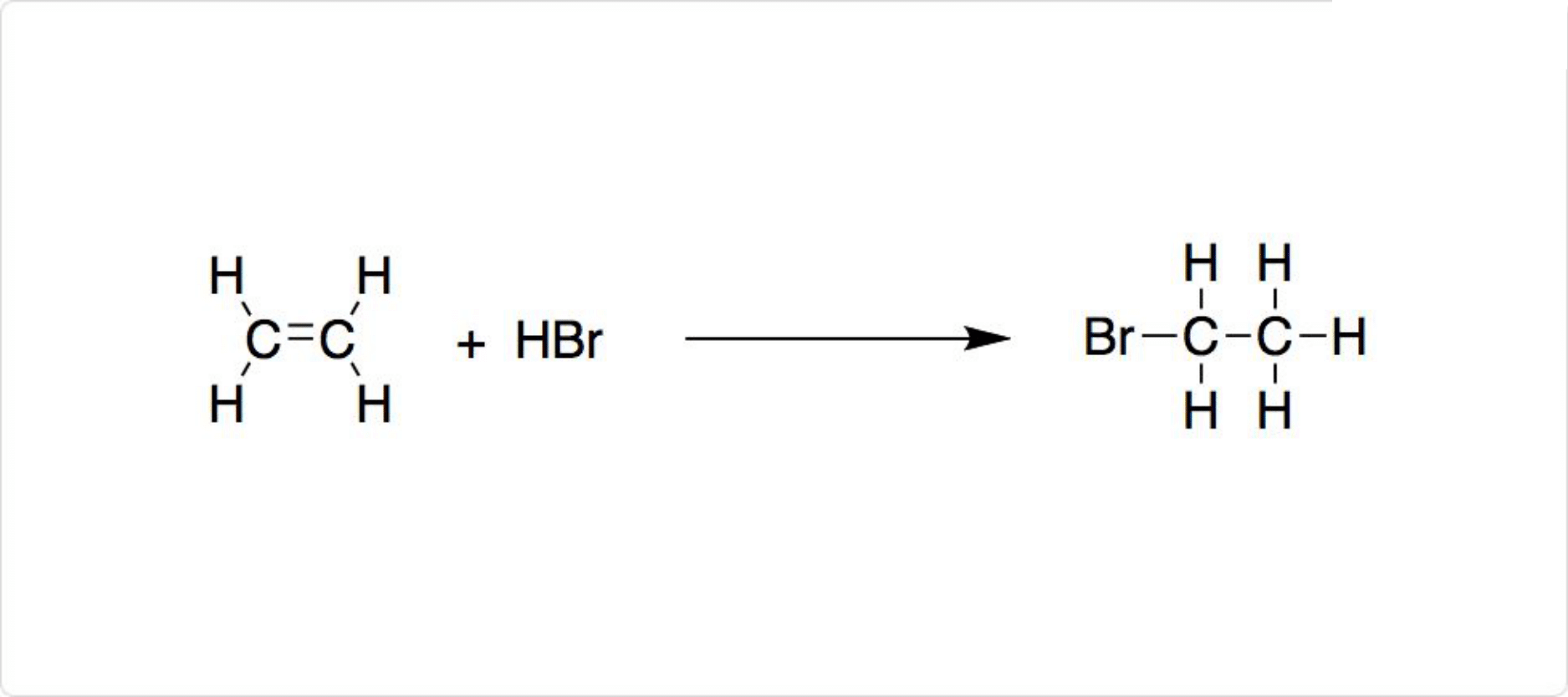
図2. 臭化エチルの合成
炭化水素の臭素化によって、臭化エチルを合成可能です。一般的に臭化エチルの合成では、エチレンに臭化水素を付加する場合が多いです。ただし臭化エチルは安価な化合物であり、通常研究室では合成しません。
また、エタノールと臭化水素酸に少量の硫酸を加えて蒸留すると、臭化エチルが生成します。この反応では副生成物として、ジエチルエーテルが生じます。さらに、エタノールを臭化チオニル (SOBr2) や三臭化リン (PBr3) と反応させても、臭化エチルを生成可能です。
-
説明
Ethyl bromide is a colorless to yellow liquid that becomes a gas
at temperatures above 101 °F (38 ℃). Ethyl bromide has an
etherlike odor and a burning taste. Ethyl bromide is miscible
with alcohols, ether, chloroform, and organic solvents; its water
solubility is 9000 mg l-1 at 25 ℃. Ethyl bromide’s vapor
density relative to air is 3.76.
-
化学的特性
Bromoethane is a colorless, volatile, flammable liquid. When exposed to air and light, it turns yellow. It has an ethereal odor and somewhat burning taste. Bromoethane has a specific gravity of 1.4505 between 4° and 25°C, a boiling point of 38.4°C, a melting point of -119°C, and a vapor pressure of 475 mm mercury at 25°C. It is 0.91% (w/w) soluble in water at 20°C and is miscible with ethanol, ethyl ether, chloroform, and other organic solvents. It has a flash point of -20°C (closed cup). The autoignition temperature is 511°C. The flammable limits in air are between 6.7570 and 11.25%. Although bromoethane is relatively stable, when heated to decomposition it emits highly toxic fumes of bromine and hydrobromide; it can react with oxidizing materials (ITII, 1979; Sittig, 1979; Torkelson and Rowe, 1981; Merck, 1983; Sax, 1984).
-
物理的性質
Clear, colorless to yellow, volatile liquid with an ether-like odor. Odor threshold concentration is
3.1 ppm (quoted, Amoore and Hautala, 1983).
-
使用
Bromoethane is an alkylating agent primarily used as a chemical intermediate in organic synthesis, in the manufacture of pharmaceuticals, and for the ethylation of gasoline. To a lesser extent, it has been used as a fruit and grain fumigant, refrigerant, and solvent. Although proposed occasionally as a general anesthetic in the earlier part of this century, it has not been used to any extent for this purpose (Sayers et al., 1929; Abreu et al., 1939; ITII, 1979; Sittig, 1979; Torkelson and Rowe, 1981;Merck, 1983).
-
定義
ChEBI: Bromoethane is a bromoalkane that is ethane carrying a bromo substituent. It is an alkylating agent used as a chemical intermediate in various organic syntheses. It has a role as a carcinogenic agent, a solvent, a refrigerant, a local anaesthetic and an alkylating agent. It is a bromoalkane, a bromohydrocarbon and a volatile organic compound.
-
製造方法
Bromoethane is produced by the reaction of either hydrogen or potassium bromide with cold ethanol or with ethylene and sulfuric acid (Hawley, 1977; Sittig, 1979; Merck, 1983). It is commercially available at greater than 99% purity. Production from two U.S. manufacturers was estimated at 163.5 million pounds in 1986 (CSITC, 1987); no recent import and export information was available in the literature.
-
一般的な説明
A colorless volatile liquid. Slightly soluble in water and denser than water. Flash point below 0°F. Vapors are heavier than air. Toxic by inhalation. Irritates skin and eyes. Used to make pharmaceuticals and as a solvent.
-
空気と水の反応
Highly flammable. Slightly soluble in water and denser than water. Turns yellow on exposure to air and light.
-
反応プロフィール
Bromoethane will react with steam to produce toxic and corrosive fumes. Bromoethane can react vigorously with oxidizers. Bromoethane reacts with strong bases. Bromoethane also reacts with chemically active metals such as sodium, potassium, calcium, powdered aluminum, zinc and magnesium. Bromoethane will attack some forms of plastics, rubber and coatings.
-
危険性
Toxic by ingestion, inhalation, and skin
absorption; strong irritant. Questionable carcinogen. Flammable, dangerous fire hazard, explosion
limits in air 6–11%.
-
健康ハザード
Ethyl bromide is a depressant of the centralnervous system, causing narcosis. The healthhazard is greater than with ethyl chloride.In addition to the narcotic effects that occurat exposure to high concentrations, othertoxic symptoms include irritation of the eyesand respiratory tract, pulmonary edema, fattydegeneration of the liver and renal tissue, anddamage to the liver, kidney, and intestine. A15-minute exposure to a 15% concentrationof vapor in air was lethal to rats. The oralLD50 value in rats is 1350 mg (NIOSH 1986).
-
火災危険
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion and poison hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
-
使用用途
臭化エチルは、有機合成のためのエチル化剤として使用されています。例えば、カルボン酸塩からのエチルエステル合成やアミン類のエチル化によるエチルアミン合成などに使われています。
また、有機マグネシウムハロゲン化物であるグリニャール試薬 (英: Grignard reagent) の原料としても使用可能です。有機合成でグリニャール試薬は、欠かせない試薬として長年使われています。
医薬分野では医薬品の原料として使われ、麻酔薬に使われる場合もあります。
-
安全性プロファイル
Confirmed carcinogen.
Moderately toxic by ingestion and
intraperitoneal routes. Mddly toxic by
inhalation. An eye and skin irritant.
Physiologically, it is an anesthetic and
narcotic. Its vapors are markedly irritating to
the lungs on inhalation for even short
periods. It can produce acute congestion
and edema. Liver and ludney damage in
humans has been reported. It is much less
toxic than methyl bromide, but more toxic
than ethyl chloride. It is a preparative
hazard. Dangerously flammable by heat,
open flame (sparks), oxidizers. Moderately
explosive when exposed to flame. Reacts
with water or steam to produce toxic and
corrosive fumes. Vigorous reaction with
oxidizing materials. To fight fire, use CO2,
dry chemical. Ready decomposes when
heated to emit toxic fumes of Br-. See also
BROMIDES.
-
職業ばく露
This chemical is used as an industrial
chemical, pharmaceutical, and veterinary drug; as an ethylating agent in organic synthesis and gasoline; as a refrigerant; and as an extraction solvent. It has limited use as a
local anesthetic
-
発がん性
This chemical is considered to
be an animal carcinogen with unknown relevance to humans.
There is no EPA (IRIS) file.
In the lifetime carcinogenic/toxicology study, groups
of rats and mice were exposed 6 h/day, 5 days/week for 104
weeks to 0, 100, 200, or 400 ppm by inhalation. Survival of
rats was unaffected or in the case of the 100 ppm female rats
was significantly above the control group. Body weights
were also unaffected.
Likewise survival of mice was little affected by exposure
except for a decrease in survival at 400 ppm in female mice,
which also had body weights 6–16% lower than controls after
29 weeks. No clinical signs were apparent in any group, but
at autopsy there was evidence of respiratory irritation at
400 ppm. It was concluded that there was clear evidence
of an increase in neoplasms (endometrial adenomas,adenocarcinomas, and squamous cell carcinomas) in the uteri
of female mice. The tumors contributed to the decreased
survival of the female mice exposed to 400 ppm. The terminal
rats of uterine tumors were 0, 3, 14, and 61% in the 0, 100,
200, and 400 ppm groups.
There was equivocal evidence of carcinogenic activity in
the lungs of male mice, and a marginally increased incidence
of neoplasms in the brain and lungs of female rats. Male
rats were considered to have some evidence of a slightly
increased incidence of tumors in the adrenals, brain, and
lungs. Although there was a clear dose–response relationship
in female mice, the dose response in male mice and rats of
both sexes was not as clear.
-
輸送方法
UN1891 Ethyl bromide, Hazard Class: 6.1;
Labels: 6.1-Poisonous materials.
-
合成方法
赤リンの存在下エタノールに臭素を徐々に作用させたあと,蒸留?精製する。触媒の存在下エチレンに臭化水素を付加させる方法もある
-
純化方法
The main impurities are usually EtOH and water, both of which form azeotropes with it. Ethanol and unsaturated compounds can be removed by washing with conc H2SO4 until no further coloration is produced. The ethyl bromide is then washed with water, aqueous Na2CO3, and water again, then dried with CaCl2, MgSO4 or CaH2, and distilled from P2O5. Olefinic impurities can also be removed by storing the ethyl bromide in daylight with elemental bromine, later removing the free bromine by extraction with dilute aqueous Na2SO3, drying the ethyl bromide with CaCl2 and fractionally distilling it. Alternatively, unsaturated compounds can be removed by bubbling oxygen containing ca 5% ozone through the liquid for an hour, then washing with aqueous Na2SO3 to hydrolyse ozonides and remove hydrolysis products, followed by drying and distillation. [Beilstein 1 IV 150.]
-
不和合性
May form explosive mixture with air.
Hydrolyzes in water, forming hydrogen bromide (HBr).
Oxidizers may cause fire or explosions. Fire and explosions
may be caused by contact with chemically active metals:
aluminum, magnesium or zinc powders; lithium, potassium,
sodium. Attacks some plastic, rubber and coatings. Note:
Chlorinating agents destroy nitrogen mustards. Dry chlorinated lime and chloramines with a high content of active
chlorine, vigorously chlorinate nitrogen mustards to the carbon chain, giving low toxicity products. In the presence of
water this interaction proceeds less actively. They are rapidly oxidized by peracids in aqueous solution at weakly
alkaline pH. In acid solution the oxidation is much slower
-
廃棄物の処理
Controlled incineration with
adequate scrubbing and ash disposal facilities.