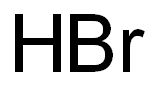
臭化水素酸
化学名:臭化水素酸
CAS番号.10035-10-6
英語名:Hydrogen bromide
CBNumberCB6852573
MFBrH
MW80.91
MOL File10035-10-6.mol
别名
臭化水素 (30%酢酸溶液, 約5.1mol/L)
臭化水素酸 - エタノール 試薬 (10-20%)
臭化水素酸 - メタノール 試薬 (5-10%)
more
臭化水素酸物理性質
| 融点 | −87 °C(lit.) |
| 沸点 | −67 °C(lit.) |
| 比重(密度) | 1.49 g/mL at 25 °C (lit.) |
| 蒸気密度 | 2.8 (vs air) |
| 蒸気圧 | 334.7 psi ( 21 °C) |
| 屈折率 | n |
| 闪点 | 40°C |
| 貯蔵温度 | Store below +30°C. |
| 溶解性 | 溶ける |
| 酸解離定数(Pka) | -9(at 25℃) |
| 外見 | 解決 |
| 色 | ライトイエロー、ブラウン |
| 比重 | 1.49 |
| 臭い (Odor) | 2ppmで検出可能な鋭い刺激臭 |
| PH | 3.01(1 mM solution);2.04(10 mM solution);1.08(100 mM solution) |
| 水溶解度 | 溶ける |
| Sensitive | Hygroscopic |
| Merck | 14,4778 |
show more
| 主な危険性 | C,Xi |
| Rフレーズ | 35-37-34-10-36/37/38 |
| Sフレーズ | 26-45-7/9-36/37/39 |
| RIDADR | UN 3265 8/PG 2 |
| OEL | Ceiling: 3 ppm (10 mg/m3) |
| WGK Germany | 1 |
| RTECS 番号 | MW3850000 |
| TSCA | Yes |
| DOT Classification | 2.3, Hazard Zone C (Gas poisonous by inhalation) |
| 国連危険物分類 | 8 |
| 容器等級 | II |
| HSコード | 28111990 |
| 有毒物質データの | 10035-10-6(Hazardous Substances Data) |
| 毒性 | LC50 in mice, rats: 814, 2858 ppm by inhalation, K. C. Back et al., Reclassification of Materials Listed as Transportation Health Hazards (TSA-20-72-3, PB 214-270, 1972) |
| IDLA | 30 ppm |
| 消防法 | 危-4-A-II |
| 化審法 | (1)-105 |
| 安衛法 | 有機則 第二種有機溶剤等 |
| 毒劇物取締法 | 劇物 |

