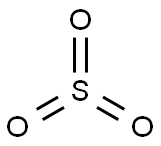
-
種類
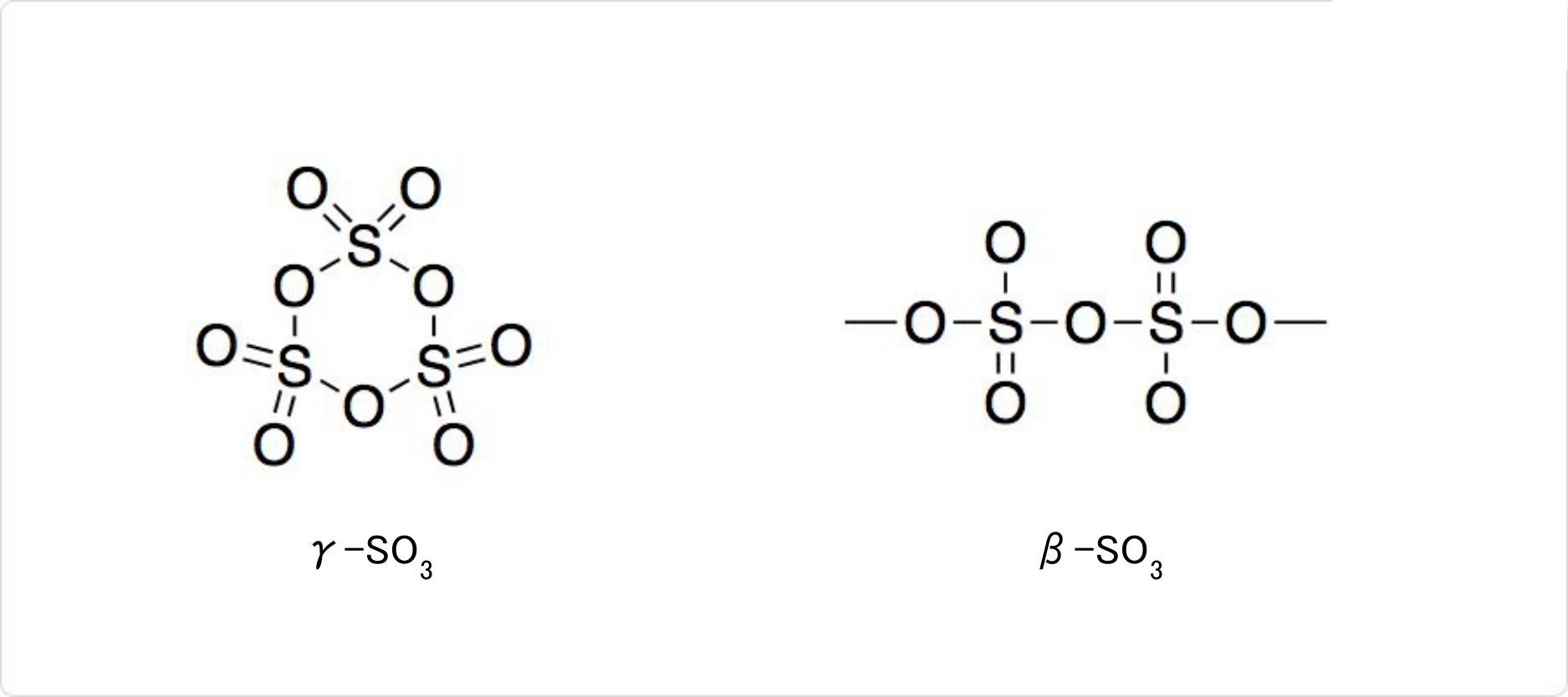
図2. 三酸化硫黄の種類
固体の三酸化硫黄にはα-SO3、β-SO3、γ-SO3の3種類の変態があります。微量の水に依存して、複雑な挙動を示します。気体が凝集して、三量体のγ-SO3を形成します。γ-SO3はS3O9と表される分子結晶です。
27°C以上で痕跡量の水の存在で凝集して、アスベストのような繊維状のα-SO3になります。封管中でβ-SO3を32.5°C以上で放置しても得られます。α-SO3は末端にヒドロキシル基を持つ[S(=O)2(μ-O)]n型の高分子です。
β-SO3もα型と同じく針状結晶です。β-SO3とγ-SO3は、微量の水によって、時間とともに安定なα-SO3に少しずつ相転移します。四面体型のSO4がOによって繋がった無限らせん構造を持っています。
-
性質
三酸化硫黄の融点は16.9°C、沸点は45°Cで、常温で無色透明の液体です。強い刺激臭があり、濃硫酸に溶かすと発煙硫酸が得られます。
三酸化硫黄の吸湿性は高いです。木や綿が熱濃硫酸に触れると発火しますが、三酸化硫黄が炭水化物中の水分を脱水し、炭水化物が燃えやすくなるからです。
-
反応
三酸化硫黄は硫酸の無水物で、水と急速な発熱反応が進行します。そのため三酸化硫黄は、酸性雨の原因物質の一つです。340°C以上では、三酸化硫黄、硫酸、水の平衡状態になります。また、二塩化硫黄と三酸化硫黄の反応によって、塩化チオニルが生じます。
-
解説
三酸化硫黄.二酸化硫黄を白金海綿,V2O5,NOなどを触媒として直接酸素と反応させてつくる.気体ではSO3分子が存在し,Sを中心とする平面三角形型構造.原子間距離S-O0.143 nm.∠O-S-O120°.腐食性気体で,水とはげしく反応して硫酸となる.金属酸化物とは熱を発して反応し硫酸塩となる.固体には三つの変態が知られている.(1)斜方晶系三酸化硫黄(γ-SO3).-80 ℃ 以下で気体を凝縮させると得られる.発煙性があり,無色の氷状の固体.融点16.8 ℃,沸点44.8 ℃.密度1.995 g cm-3(13 ℃),1.97 g cm-3(20 ℃).環状の三量体でS3O9分子からなる分子結晶.(2)絹糸光沢のアスベスト状三酸化硫黄(β-SO3).25 ℃ 以下で痕跡の水の存在で斜方晶系SO3または液体SO3を放置すると得られる.融点32.5 ℃.四面体型のSO4のOでつながった無限らせん構造である.(3)もっとも安定なアスベスト状三酸化硫黄(α-SO3).β-SO3を封管中で32.5 ℃ 以上に長くおくと得られる.融点62.2 ℃.液体のSO3は単量体と三量体の混合物で,ホウ酸を添加すると安定化し,純粋な状態では痕跡の水でたやすく重合する.強酸化剤,スルホン化剤,イオン交換樹脂の製造,硫酸製造などに用いられる.猛毒.
-
用途
合成洗剤用アルキルベンゼン,高級アルコール,エチレンオキシド付加物などのスルホン化および硫酸化,石油潤滑留分および芳香族炭化水素のスルホン化,クロロスルホン酸の合成,スルホンアミドの合成などに利用されている。
-
構造

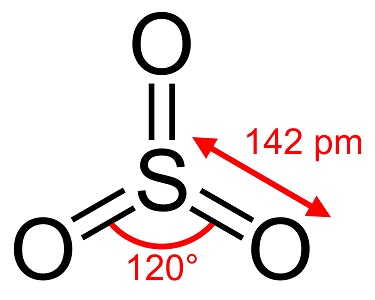
図1. 三酸化硫黄の構造
三酸化硫黄のモル質量は80.06g/mol、密度は1.92g/cm3です。
気体の三酸化硫黄は、硫黄原子が中心の平面正三角形構造を取っていると予測されています。硫黄原子の酸化数は+6であり、電荷は0で、電子対を6個有します。分子軌道法によると、電子対の多くは非結合的な性質を有する典型的な超原子価分子です。3個のS-Oの長さは等しく、1.42Åであり、∠O-S-Oは120°です。
-
合成法
実験室で三酸化硫黄は、硫酸水素ナトリウムの熱分解によって合成されます。ナトリウム以外の金属の硫酸水素塩でも反応は進行しますが、中間体の安定性に反応条件は依存します。
工業的に接触法で、三酸化硫黄を製造可能です。まず、硫黄や黄鉄鉱の燃焼によって、二酸化硫黄を合成して、電気集塵によって精製します。続いて、二酸化硫黄を酸素や五酸化バナジウムを400~600°Cに加熱して酸化すると、三酸化硫黄を合成可能です。
鉛室法の過程でも、二酸化硫黄と二酸化窒素の反応によって、三酸化硫黄が生じます。鉛室法とは、硝酸類や窒素酸化物を使用した硫酸の製造法のことです。接触法が登場したために、現在では鉛室法は廃れてしまいました。
-
化学的特性
Sulfur trioxide, S03, also known as sulfuric anhydride, needles or polymer, exists in a number of modifications that differ in molecular species and crystalline form. It has a white,ice-like modification that melts at 16°C (61°F) and two other as bestos-like forms that melt at the higher temperatures of 33 and 62°C (90 and 144 °F). The colorless liquid or gas form has irritating, toxic fumes and boils at 45 °C (112 °F).
Sulfur trioxide is a highly reactive substance, a strong oxidizing agent,and a fire hazard. It reacts with metallic oxides to form sulfates and with water to form sulfuric acid. Sulfur trioxide is used for sulfonation.
-
物理的性質
Colorless liquid at ambient temperature and atmospheric pressure; fumes in air.
Sulfur trioxide tends to polymerize, particularly in the presence of traces of water or sulfuric acid. The rate of its polymerization, however, decreasesgreatly as its freezing point is approached. Solid (polymeric) sulfur trioxide exists in three polymorphic phases: alpha-, beta- and gamma- modifications.
The alpha phase is made up of ice-like needles having polymeric crosslinked structure. It melts at 62.3°C and has a vapor pressure of 73 torr at 25°C.
The beta phase is a metastable allotrope with white, asbestos-like, lustrous needles consisting of polymeric molecules, melting at 32.5°C, and with vapor pressure 344 torr at 25°C.
The gamma modification at ordinary temperatures can exist in solid or liquid form. In solid form it is a colloidal ice-like mass melting at 16.8°C. In the liquid form it has a density of 1.9224 g/mL, boiling at 44.8°C. It has a vapor pressure of 433 torr at 25°C. The gamma phase consists of both cyclic trimer and monomer molecules. When solid sulfur trioxide melts, it converts to its gamma phase which on solidification changes to alpha modification.
Critical temperature of SO3 is 217.8°C; critical pressure 80.97 atm; critical density 0.63 g/cm3; the dielectric constant of liquid SO3 at 18°C is 3.11.
Sulfur trioxide dissolves in water forming sulfuric acid and generating large heat.
-
使用
Sulfur trioxide is used as an intermediate in the manufacture of sulphuric acid and oleum for sulphonation, in particular, of dyes and dye-stuffs, and for the production of anhydrous nitric acid and explosives. Solid Sulfur trioxide is marketed under such names as Sulphan and Triosul, and is used primarily for sulphonation of organic acids. Sulphur tetrafluoride is a fluorinating agent. Sulphur hexafluoride serves as a gaseous insulator in high-voltage electric installations. Sulphyryl fluoride is used as an insecticide and a fumigant.
Sulfonation of organic compounds, especially nonionic detergents, solar energy collectors. It is usually generated in the plant where it is to be used.
-
調製方法
Sulfur trioxide is produced as an intermediate in manufacturing sulfuric acid by the contact process (See Sulfuric Acid). The process involves catalytic oxidation of sulfur dioxide to trioxide.
Sulfur trioxide is prepared in the laboratory by heating fuming sulfuric acid, condensing its vapors, and collecting in a cool receiver. When vapors are condensed below 27°C in the presence of trace moisture, all three polymorphic phases of SO3 are produced. They can be separated by fractional distillation. Condensation of the vapors above 27°C forms the liquid variety of gamma-sulfur trioxide.
-
一般的な説明
Sulfur trioxide, is a colorless to white crystalline solid which will fume in air. Often shipped with inhibitor to prevent polymerization. Sulfur trioxide reacts violently with water to form sulfuric acid with the release of heat. Sulfur trioxide is corrosive to metals and tissue. Sulfur trioxide causes eye and skin burns. Ingestion causes severe burns of mouth esophagus and stomach. The vapor is very toxic by inhalation. Sulfur trioxide is a fire risk when in contact with organic materials such as wood, cotton, fiberboard, etc.
-
空気と水の反応
Combines with water with explosive force, forming sulfuric acid due to its acidity Sulfur trioxide chars most organic substances. On exposure to air Sulfur trioxide absorbs moisture rapidly, emitting dense white fumes [Merck 11th ed. 1989].
-
反応プロフィール
The reaction of Sulfur trioxide and oxygen difluoride is very vigorous and explosions occur if the reaction is carried out in the absence of a solvent [J. Chem. Eng. Data 13(4):529-531. 1968]. The reaction of Sulfur trioxide in excess with tetrafluoroethylene causes explosive decomposition to carbonyl fluoride and sulfur dioxide [Chem. Eng. News 49(22):3. 1971]. The reaction of anhydrous perchloric acid with Sulfur trioxide is violent and accompanied by the evolution of considerable heat (Pascal 16:300 1931-34). Liquid Sulfur trioxide reacts violently with nitryl chloride, even at 75° C. The reaction of Sulfur trioxide and lead oxide causes white luminescence [Mellor 7:654 1946-47]. The combination of iodine, pyridine, Sulfur trioxide, and formamide developed a gas over pressurization after several months. This is due to the slow formation of sulfuric acid, from external water or dehydration of the formamide to hydrogen cyanide.
-
危険性
Oxidizing agent, fire risk in contact with
organic materials, an explosive increase in vapor
pressure occurs when the α form melts. The anhydride
combines with water, forming sulfuric acid
and evolving heat. Highly toxic, strong irritant to
tissue.
-
健康ハザード
Sulfur trioxide is highly toxic. It is an irritant and corrosive to mucous membranes. Poisonous if inhaled or swallowed. Contact causes severe burns to skin and eyes.
-
火災危険
Fire risk in contact with organic materials. An explosive increase in vapor pressure occurs when the alpha form melts. Combines with water with explosive violence, forming sulfuric acid. May ignite other combustible materials (wood, paper, oil, etc.). Flammable poisonous gases may accumulate in tanks and hopper cars. Runoff to sewer may create fire or explosion hazard. Forms sulfuric acid on contact with water. Avoid water and organic materials. On exposure to air, Sulfur trioxide absorbs moisture and emits dense white fumes.
-
使用用途
三酸化硫黄は、酸化剤、スルホン化剤、の製造、硫酸の製造などに使用されます。スルホン化剤は工業的な有機合成で広く用いられており、染料や中性洗剤などの工業的な製造工程に使用可能です。
三酸化硫黄は接触法の中間体でもあります。硫黄と酸素から製造したを触媒存在下で三酸化硫黄とし、発煙硫酸を経由して水と反応させると、高純度な濃硫酸を製造できます。
-
化学性质
刺激臭がある。α,β,γの3種の変態があり,安定化液体三酸化硫黄はγ型に安定化したものである。硫酸,発煙硫酸より強力な脱水作用を有し,金属に対する腐食性は小さい。有機化合物と反応するとき,水を生成することなく,スルホン基を導入することができる。
-
安全性プロファイル
Poison by inhalation. Human systemic effects by inhalation: cough and other pulmonary and olfactory changes. A corrosive irritant to skin, eyes, and mucous membranes. Violent reaction with O2F2, PbO, NClO2, HClO4, P, tetrafluorethylene, acetonitrile, sulfuric acid, dimethyl sulfoxide, dioxan, water, diphenylmercury, formamide, iodine, pyridine, metal oxides. Reacts with steam to form corrosive, toxic fumes of sulfuric acid. When heated to decomposition it emits toxic fumes of SO,. See also SULFURIC ACID.
-
職業ばく露
Sulfur trioxide is used as a sulfating and sulfonating agent for detergent, lubricating oil additives, and other organic compounds; in solar energy collectors. It is also used as an intermediate in sulfuric acid manufacture and in making explosives.
-
貯蔵
The vapour pressure of Sulfur trioxide rises rapidly with increasing temperatures and, when the α-form melts, the pressure rise is explosive; consequently transport and storage containers must withstand pressures of 10 to 15 atm. Sulfur trioxide reacts vigorously and highly exothermically with water to produce hydrosulphuric acid. When exposed to moist air, it fumes and forms a mist of sulphuric acid which eventually fills all the available space; it also corrodes metals. It is a powerful oxidizing agent and, in the liquid phase, carbonizes organic materials.
-
輸送方法
UN1829 Sulfur trioxide, stabilized, Hazard class: 8; Labels: 8-Corrosive material, 6.1-Poisonous Inhalation Hazard, Inhalation Hazard Zone B.
-
合成方法
硫酸プラント,重油燃焼装置などの排ガス中の二酸化硫黄を水酸化ナトリウムで処理して回収する方法がある。
-
不和合性
Combustible and Corrosive. A strong oxidizer. Reacts violently with water, steam or moisture, releasing corrosive hydrosulfuric acid. Violent reactions occur on contact with strong bases; strong acids, chemically active metals; reducing agents; finely divided metal; cyanides, nitrates, picrates, fulminates, chlorates, sulfides, carbides, phosphorus, dioxygen difluoride, barium oxide; lead oxide; diphenyl mercury; alcohols, nitryl chloride; acetonitrile, dioxane, tetrafluoroethylene.
-
廃棄物の処理
Return refillable compressed gas cylinders to supplier. Nonrefillable cylinders should be disposed of in accordance with local, state and federal regulations. Allow remaining gas to vent slowly into atmosphere in an unconfined area or exhaust hood. Refillabletype cylinders should be returned to original supplier with any valve caps and outlet plugs secured and valve protection caps in place.