-
外観
白色~わずかにうすい黄色, 結晶性粉末~粉末
-
性質
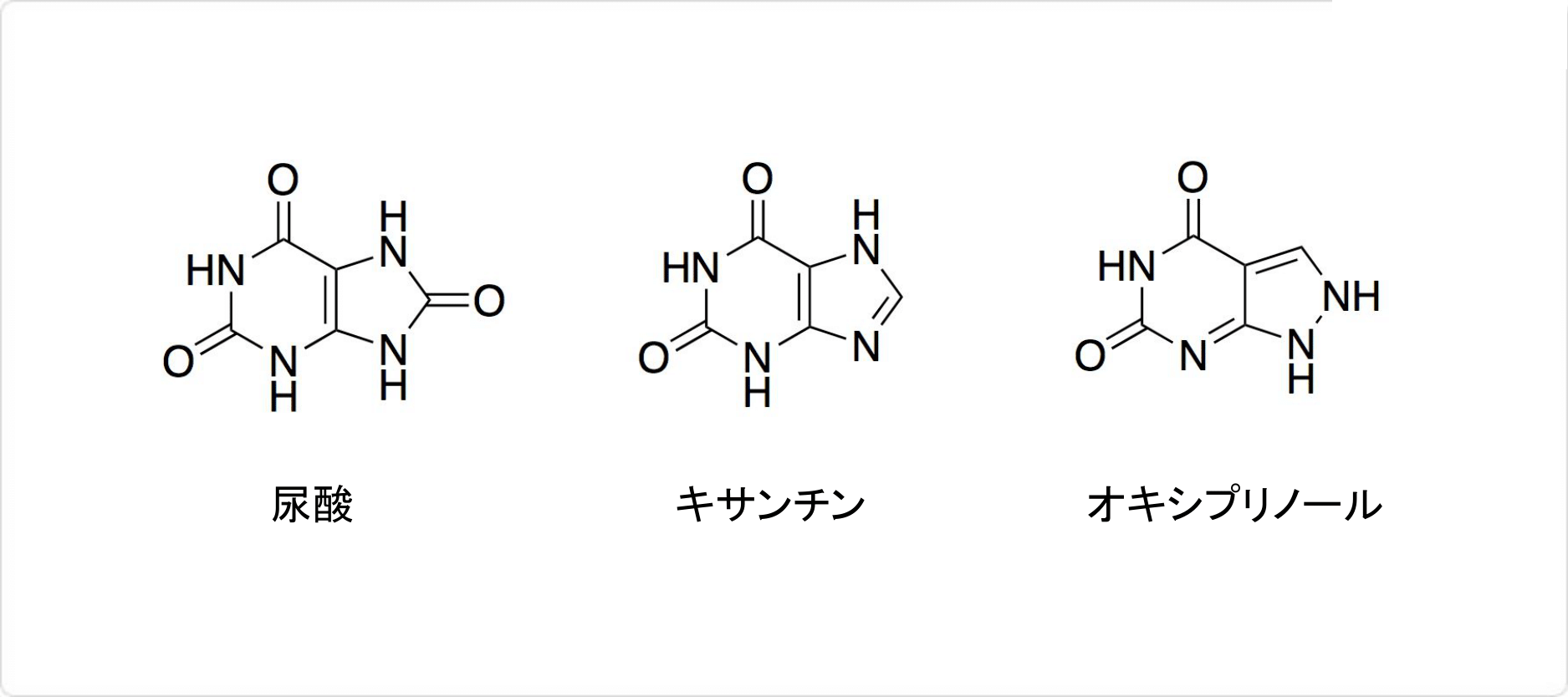
図2. アロプリノールの関連化合物
アロプリノールの化学式はC5H4N4Oで、分子量は136.112g/molです。天然に存在するプリン (英: purine) 誘導体の一つであるヒポキサンチンの構造異性体です。外観は白色の結晶性で粉末です。アンモニア試液には溶けますが、水やには溶けにくいです。
体内に入ったアロプリノールは、尿酸の合成を阻害します。体内では酵素であるキサンチンオキシダーゼの働きによりへ代謝されています。キサンチンオキシダーゼをアロプリノールが阻害し、体内でプリン体からヒポキサンチン (英: hypoxanthine) やキサンチン (英: xanthine) を経て、尿酸 (英: uric acid) が生じるのを抑制可能です。
アロプリノールは体内ですぐにオキシプリノール (英: OxipurinolまたはOxypurinol) に代謝されます。生物学的半減期は1時間ほどと短いのに対して、オキシプリノールの半減期は17~30時間程度です。したがって、アロプリノールを投与した際の効能は、オキシプリノールによると考えられます。
-
溶解性
水に極めて溶けにくく、エタノール(95)にほとんど溶けない。
-
解説
高尿酸血症、痛風、尿路結石の治療薬で、化学的にはヒポキサンチンの立体異性体である。低濃度では競合的に、高濃度では非競合的にキサンチンオキシダーゼを阻害する。その結果、血中および尿中の尿酸値が著明に低下する。1日0.2~0.3グラム内服する。劇薬で、極量は1回0.2グラム、1日0.6グラム。
小学館 日本大百科全書(ニッポニカ) )
-
用途
キサンチンオキシダーゼを阻 害し、尿酸生成抑制作用を示します。
-
用途
体内での尿酸の産生を抑制し、高尿酸血症を改善することにより痛風発作の発生を予防する。
-
用途
薬理研究用。
-
用途
アロプリノール(Allopurinol)は痛風?高尿酸血症治療薬。ヒポキサンチンの構造異性体で、キサンチンオキシダーゼの阻害活性を有する。体内での尿酸の産生を抑制し、高尿酸血症を改善することにより痛風発作の発生を予防する。
-
効能
痛風治療薬, 尿酸生合成抑制薬, キサンチンオキシダーゼ阻害薬
-
商品名
アロプリノール (ナガセ医薬品); ザイロリック (グラクソ・スミスクライン)
-
化学的特性
White to Off-White Solid
-
使用
Allopurinol does not reduce serum uric acid levels by increasing renal uric acid excretion; instead it lowers plasma urate levels by inhibiting the final steps in uric acid biosynthesis.
Uric acid in humans is formed primarily by xanthine oxidase-catalyzed oxidation of hypoxanthine and xanthine to uric acid. Allopurinol (8) and its primary metabolite, alloxanthine (9) [CAS: 2465-59-0], are inhibitors of xanthine oxidase. Inhibition of the last two steps in uric acid biosynthesis by blocking xanthine oxidase reduces the plasma concentration and urinary excretion of uric acid and increases the plasma levels and renal excretion of the more soluble oxypurine precursors. Normally, in humans the urinary purine content is almost solely uric acid; treatment with allopurinol results in the urinary excretion of hypoxanthine, xanthine, and uric acid, each with its independent solubility. Lowering the uric acid concentration in plasma below its limit of solubility facilitates the dissolution of uric acid deposits. The effectiveness of allopurinol in the treatment of gout and hyperuricemia that results from hematogical disorders and antineoplastic therapy has been demonstrated.
-
適応症
Allopurinol (Zyloprim) is the drug of choice in the treatment
of chronic tophaceous gout and is especially useful
in patients whose treatment is complicated by renal insufficiency.
-
一般的な説明
Odorless tasteless white microcrystalline powder.
-
空気と水の反応
Insoluble in water.
-
反応プロフィール
Allopurinol is an aminoalcohol. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. Allopurinol darkens above 572° F, and at an indefinite high temperature, Allopurinol chars and decomposes. At 221° F, maximum stability occurs at pH 3.1- 3.4. Allopurinol decomposes in acidic and basic solutions.
-
火災危険
Flash point data for Allopurinol are not available; however, Allopurinol is probably combustible.
-
使用用途
アロプリノールは、体内での尿酸の生成を抑えるため、高尿酸血症の治療に用いることが可能です。通常、成人は1日200mgから300mgを、2回から3回に分けて経口摂取します。ただし摂取初期は、尿酸の結晶が関節から排出されるため、痛風発作が起こることもあります。
主な副作用に、発疹、かゆみ、関節痛、貧血、肝機能異常、食欲不振、軟便、下痢、倦怠感、脱毛があるため注意が必要です。アロプリノールは腎臓から排出されるので腎臓機能が低下していると薬の排出が遅れがちになるため、摂取量を調整する必要があります。
-
関連化合物
アロプリノールの関連化合物
 図3. アロプリノールの構造異性体
図3. アロプリノールの構造異性体
ヒポキサンチンは、アロプリノールの構造異性体です。ヒポキサンチンは核酸で見られるプリン誘導体であり、ヌクレオシドイノシンとしてtRNAのアンチコドンに存在します。
キサンチンオキシダーゼによりキサンチンからヒポキサンチンが生成し、サルベージ経路 (英: salvage pathway) のヒポキサンチン-グアニンホスホリボシルトランスフェラーゼ (英: Hypoxanthine-guanine phosphoribosyltransferase) によりイノシン一リン酸 (英: inosinic acid) に変わります。
-
作用機序
Allopurinol, in contrast to the uricosuric drugs, reduces
serum urate levels through a competitive inhibition of
uric acid synthesis rather than by impairing renal urate
reabsorption. This action is accomplished by inhibiting
xanthine oxidase, the enzyme involved in the metabolism
of hypoxanthine and xanthine to uric acid. After
enzyme inhibition, the urinary and blood concentrations
of uric acid are greatly reduced and there is a simultaneous
increase in the excretion of the more soluble
uric acid precursors, xanthine and hypoxanthine.
Allopurinol itself is metabolized by xanthine oxidase
to form the active metabolite oxypurinol, which
tends to accumulate after chronic administration of the
parent drug.This phenomenon contributes to the therapeutic
effectiveness of allopurinol in long-term use.
Oxypurinol is probably responsible for the antigout effects
of allopurinol. Oxypurinol itself is not administered
because it is not well absorbed orally.
-
薬物動態学
Allopurinol was synthesized in 1956 as part of a study of purine antagonists. It is well absorbed on oral
administration, with peak plasma concentrations appearing within 1 hour. Decreases of uric acid can be observed
within 24 to 48 hours. Excretion of allopurinol and its metabolite occurs primarily in the urine, with approximately 20%
of a dose being excreted in the feces.
-
臨床応用
Allopurinol is especially indicated in the treatment of
chronic tophaceous gout, since patients receiving it show
a pronounced decrease in their serum and urinary uric
acid levels. Because it does not depend on renal mechanisms
for its efficacy, allopurinol is particularly beneficial
for patients who already have developed renal uric acid
stones, patients with excessively high urate excretion
(e.g., above 1,200 mg in 24 hours), patients with a variety
of blood disorders (e.g., leukemia, polycythemia vera),
patients with excessive tophus deposition, and patients
who fail to respond well to the uricosuric drugs.
Allopurinol also inhibits reperfusion injury. This injury
occurs when organs that either have been transplanted
or have had their usual blood perfusion blocked
are reperfused with blood or an appropriate buffer solution.
The cause of this injury is local formation of free
radicals, such as the superoxide anion, the hydroxyl free
radical, or peroxynitrite. These substances are strong
oxidants and are quite damaging to tissues.
-
副作用
Common toxicities associated with allopurinol administration
include a variety of skin rashes, gastrointestinal
upset, hepatotoxicity, and fever. These reactions are often
sufficiently severe to dictate termination of drug
therapy. It is advised that therapy not be initiated during
an acute attack of gouty arthritis. As with the uricosuric
drugs, therapy with allopurinol should be accompanied
both by a sufficient increase in fluid intake to
ensure water diuresis and by alkalinization of the urine.
Prophylactic use of colchicine also helps to prevent
acute attacks of gout that may be brought on during the
initial period of allopurinol ingestion.
-
安全性プロファイル
Human poison by
ingestion. Poison experimentally by
intraperitoneal and subcutaneous routes. An
experimental teratogen. Human systemic
effects by ingestion: blood leukopenia,
dermatitis, jaundice, muscle weakness,
thrombocytopenia. When heated to
decomposition it emits toxic fumes of NOx.
An FDA proprietary drug used as a xanthine
oxidase inhibitor.
-
代謝
Allopurinol is rapidly metabolized via oxidation and the formation of numerous ribonucleoside derivatives. The major oxidation metabolite, alloxanthine or oxypurinol, has a much longer half-life
(18–30 hours versus 2–3 hours) than the parent drug and is an effective, although less potent, inhibitor of xanthine
oxidase. The longer plasma half-life of alloxanthine results in an accumulation in the body during chronic
administration, thus contributing significantly to the overall therapeutic effects of allopurinol.
-
予防処置
Since allopurinol is metabolized by the hepatic microsomaldrug-metabolizing enzymes, coadministration ofdrugs also metabolized by this system should be donewith caution. Because allopurinol inhibits the oxidationof mercaptopurine and azathioprine, their individualadministered doses must be decreased by as much as75% when they are given together with allopurinol.Allopurinol may also increase the toxicity of other cytotoxicdrugs (e.g., vidarabine). The actions of allopurinolare not antagonized by the coadministration of salicylates.
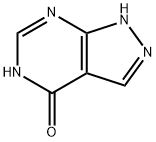


 図3
図3