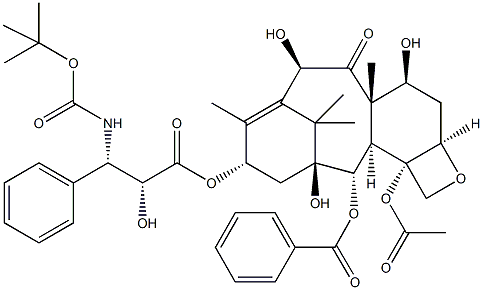
ドセタキセル
化学名:ドセタキセル
CAS番号.114977-28-5
英語名:Docetaxel
CBNumberCB1163212
MFC43H53NO14
MW807.88
MOL File114977-28-5.mol
别名
ドセタキセル水和物
ドセタキセル
N-デベンゾイル-N-tert-ブトキシカルボニル-10-デアセチルタキソール
more
ドセタキセル物理性質
| 融点 | 186-192 °C (dec.) |
| 比旋光度 | -36 º (c=0.74,EtOH) |
| 沸点 | 900.5±65.0 °C(Predicted) |
| 比重(密度) | 1.38 |
| 貯蔵温度 | Sealed in dry,Store in freezer, under -20°C |
| 溶解性 | 水にほとんど溶けず、無水エタノールに溶けやすく、塩化メチレンに溶ける。 |
| 外見 | Solid |
| 酸解離定数(Pka) | 11.20±0.46(Predicted) |
| 色 | 白い |
| 水溶解度 | ジメチルスルホキシド、エタノールに可溶。水に不溶。 |
| Merck | 14,3397 |
| 安定性: | -20°C の DMSO またはエタノール溶液で最大 3 か月間保存できます。 |
| InChIKey | BEDLLNJKXXVTSX-LWWLJZAUSA-N |
| LogP | 2.456 (est) |
| CAS データベース | 114977-28-5(CAS DataBase Reference) |
| 主な危険性 | Xi |
| Rフレーズ | 36/37/38-61 |
| Sフレーズ | 26-36/37-53-45 |
| RIDADR | 1544 |
| WGK Germany | 3 |
| RTECS 番号 | DA4172750 |
| 国連危険物分類 | 6.1(b) |
| 容器等級 | III |
| HSコード | 29322090 |
| 有毒物質データの | 114977-28-5(Hazardous Substances Data) |
| 毒性 | LD50 intravenous in dog: 2500ug/kg |

