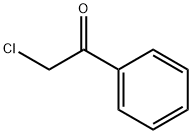
2-Chloroacetophenone
- Product Name2-Chloroacetophenone
- CAS532-27-4
- CBNumberCB9387386
- MFC8H7ClO
- MW154.59
- EINECS208-531-1
- MDL NumberMFCD00000933
- MOL File532-27-4.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 54-56 °C(lit.) |
| Boiling point | 244-245 °C(lit.) |
| Density | 1.324 g/mL at 25 °C(lit.) |
| vapor pressure | 4 at 20 °C, 14 at 30 °C (quoted, Verschueren, 1983) |
| refractive index | 1.5438 |
| Flash point | 118°C |
| solubility | Soluble in acetone (Weast, 1986). Freely soluble in alcohol, benzene, and ether (Windholz et al., 1983) |
| form | Colorless crystals |
| Water Solubility | insoluble. <0.1 g/100 mL at 19 ºC |
| Sensitive | Moisture Sensitive |
| Merck | 14,2115 |
| BRN | 507950 |
| Exposure limits | NIOSH REL: TWA 0.3 mg/m3 (0.05 ppm), IDLH 15 mg/m3; OSHA PEL: TWA 0.05 ppm (0.3 mg/m3); ACGIH TLV: TWA 0.05 ppm (adopted). |
| Stability | May decompose upon exposure to air or water. Incompatible with bases, amines, alcohols. |
| InChIKey | IMACFCSSMIZSPP-UHFFFAOYSA-N |
| LogP | 1.724 (est) |
| Toxicology and Carcinogenesis | Toxicology and Carcinogenesis Studies of 2-Chloroacetophenone (CASRN 532-27-4) in F344/N Rats and B6C3F1 Mice (Inhalation Studies) |
| CAS DataBase Reference | 532-27-4(CAS DataBase Reference) |
Safety
| Symbol(GHS) |
  
|
|||||||||
| Signal word | Danger | |||||||||
| Hazard statements | H300-H311+H331-H315-H318-H334-H335 | |||||||||
| Precautionary statements | P261-P280-P301+P310-P302+P352+P312-P304+P340+P311-P305+P351+P338 | |||||||||
| Hazard Codes | T | |||||||||
| Risk Statements | 25-36/37/38-42/43-23/25-36-23/24/25 | |||||||||
| Safety Statements | 22-26-36/37/39-45-28A-7/9 | |||||||||
| RIDADR | UN 1693 6.1/PG 2 | |||||||||
| OEB | C | |||||||||
| OEL | TWA: 0.3 mg/m3 (0.05 ppm) | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | AM6300000 | |||||||||
| F | 9-19 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| Hazardous Substances Data | 532-27-4(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 in rats (mg/kg): 41 i.v., 36 i.p., 127 orally; LC50 in rats: 8750 mg/min/m3 (Ballantyne, Swanston) | |||||||||
| IDLA | 15 mg/m3 | |||||||||
| NFPA 704: |
|


