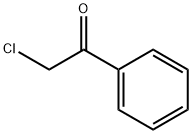
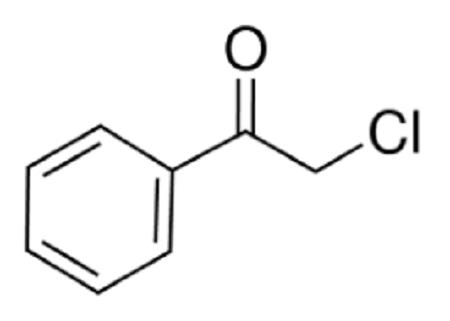
Uses of Chloroacetophenone
Chloroacetophenone(CN) was first synthesized in 1870 by the Germans. It is used as a riot control agent (RCA) due to its potency as a lachrymatory agent. It got its fame after World War I when it was given the trade name Mace, the first American manufacturer of CN devices and sold for personal and commercial protection. Generically, it is known as tear gas. It was used in US military systems until its replacement by CS (ochlorobenzylidene malononitrile) in 1959. However, CN is still sold commercially in the United States and is used by militaries and police all over the world.
Uses
Chloroacetophenone is used as a nonlethal or less-than-lethal chemical in riot control situations to distract, deter, incapacitate, disorient, or disable disorderly people; to clear facilities or areas; to deny areas; or for hostage rescue. It can also be used in peacekeeping operations. It is also used in military training as a confidence builder for the protective mask.
Environmental Fate
Chloroacetophenone has a half-life under aerobic conditions of 672 h and can be
biodegraded in most moist, nutrient-rich soil.
Biodegradation in water is similar to degradation in soil.
Howard et al. (1991) reports the same 672 h half-life for CN in
aerobic surface water. This half-life is extended up to 2688 h for
anaerobic aqueous degradation.
Volatile losses of CN from surface waters can be another
significant process for CN disappearance in water. For instance,
Olajos and Stopford (2004) calculated that CN contamination
in a river would have a half-life of approximately 14 days, while
CN contamination in a lake would have a half-life of approximately
110 days.
Mechanism of Toxicity
Chloroacetophenone is considered less than lethal or nonlethal because it has a large safety ratio. That is, its effective dose or concentration ECt50 is low compared to its lethal dose or concentration (LCt50). In the body, CN is converted to an electrophilic metabolite. It is an SN2 alkylating agent that reacts with SH groups and other nucleophilic sites of biomolecules. Alkylation of SH-containing enzymes leads to enzyme inhibition with disruption of cellular processes. CN was found to inhibit human plasma cholinesterase via a non-SH interaction, and some of the toxic effects may be due to alkylation of SHcontaining enzymes.
Chloroacetophenone as well as CS is an SN2-alkylating agent with activated
halogen groups that react readily at nucleophilic sites. The
prime targets include sulfhydryl-containing enzymes such as
lactic dehydrogenase. Alkylation of SH-containing enzymes
leads to enzyme inhibition with disruption of cellular
processes. It has been suggested that tissue injury may be
related to inactivation of certain of these enzyme systems. The
initial response to the inhalation of CN or other sensory irritants
is consistent with the Kratschmer reflex and the Sherrington
pseudoaffective response. These aerosols stimulate the
pulmonary irritant receptors to produce bronchoconstriction
and increased pulmonary blood volume by augmenting
sympathetic tone. The chlorine atoms released from CN on
contact with skin and mucous membranes are reduced to
hydrochloride acid, which can cause local irritation and burns.
CN was also found to inhibit human plasma cholinesterase via
a non-SH interaction.
Lastest Price from 2-Chloroacetophenone manufacturers

US $15.00-10.00/KG2021-07-13
- CAS:
- 532-27-4
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton

US $15.00-10.00/KG2021-07-10
- CAS:
- 532-27-4
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton