Physical properties
bp 150–153°C,bp 54–55°C/45 mmHg;
d 0.872 gmL?1
Uses
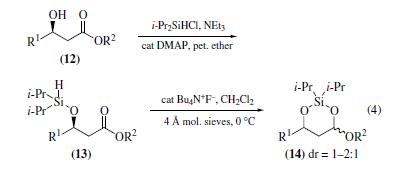
Intramolecular
hydrosilylation is also possible within β-diisopropylsilyloxy
esters (13), constituting an exceptionally mild method for reducing
ester groups to the aldehyde oxidation level (eq 4).The derivatives
(13) may be synthesized from β-hydroxy esters (12) as described
above for the analogous ketones. Treatment with fluoride
ions (but not Lewis acids) induces hydride transfer to give
alkoxysiladioxanes (14) in excellent yields (≥95%). Although
usually performed in dichloromethane, the hydrosilylation may
also be accomplished with ethyl acetate as solvent, providing
strong evidence for intramolecularity.
Application
Used in a silylation-reduction-allylation sequence of
β-hydroxy esters to homoallylic-substituted 1,3-diols.
Used in the silylation-hydrosilation-oxidation of allyl
alcohols to 1,3-diols. Reaction carried out in
diastereoselective manner. Reduces β-hydroxy ketones to
anti-1,3 diols.
Preparation
obtained by reaction of trichlorosilane with
isopropylmagnesium chloride;the original yield of 45% may
be raised to 70–80% by employing conc hydrochloric acid to
quench the reaction.
Purification Methods
Impurities can be readily detected by 1H NMR. Purify it by fractional distillation [Gilman & Clark J Am Chem Soc 69 1499 1947, Allen et al. J Chem Soc 3668 1957].