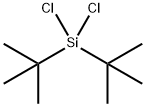
Physical properties
mp ?15°C; bp 190°C/729 mmHg; d
1.009 g cm?3.
Uses
Reagent used for preparing siliranes and protecting diols.
Uses
The presence of the bulky t-butyl
groups in di-t-butyldichlorosilane has been found to increase the
Si–C bond lengths slightly and to widen the CSiC bond angles by
11.1? relative to dichlorodimethylsilane as determined by electron
diffraction and molecular mechanics calculations.
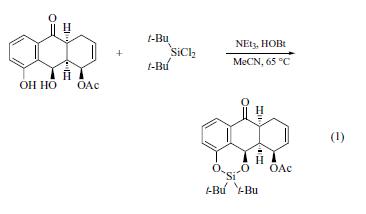
The di-t-butylsilylene protecting group for diols was introduced
by Trost and Caldwell and used in a total synthesis of
deoxypillaromycinone.It is introduced by the reaction of di-tbutyldichlorosilane
with a 1,2- or 1,3-diol in acetonitrile in the
presence of triethylamine and 1-hydroxybenzotriazole (HOBt)
at 45–90°C (eq 1). For a related, highly reactive reagent see
di-t-butylsilyl Bis(trifluoromethanesulfonate).
Preparation
can be conveniently prepared by chlorination
of di-t-butylsilane (CCl4/PdCl2 (cat), 85%)but various
other methods of preparation have been reported.
Purification Methods
Purify it by fractional distillation. 20 1.01. It is a colourless liquid with a pleasant odour and does not fume in moist air, but does not titrate quantitatively with excess of dilute alkali. [Tyler et al. J Am Chem Soc 70 2877 1948.] Di -tert-butyl silyl bis(trifluoromethanesulfonate) [85272 -31 -7] M 440.5, b 73.5 -7 4 . 5o/0.35mm, d 4 Purify it by fractional distillation at high vacuum. 20 1.36 (see pK for triflic acid). It is a pale yellow liquid which should be stored under argon. It is less reactive than the diisopropyl analogue. The presence of the intermediate monochloro compound can be detected by 1H NMR, (CHCl3): tert-Bu2Si(OTf)2 [ 1.25s], but impurities have 1.12s for tert-Bu2Si(H)OTf and 1.19s for tert-Bu2HSi(Cl)OTf. [Deslongchamps Tetrahedron Lett 23 4871 1982, Deslongchamps Aldrichimica Acta 17 72 1984.] TOXIC.