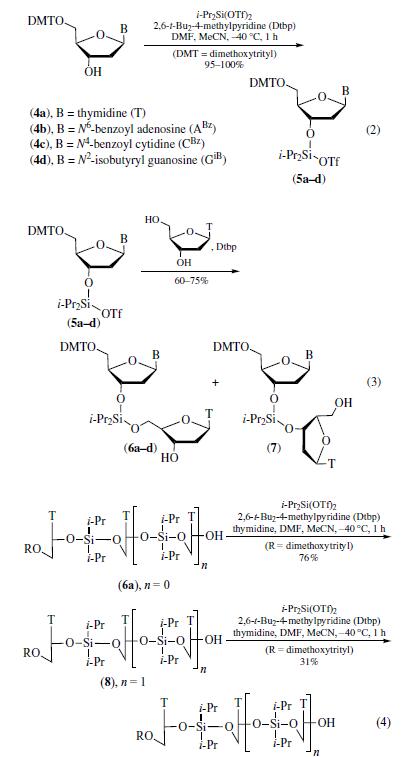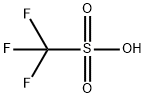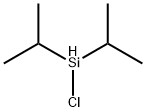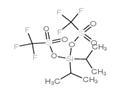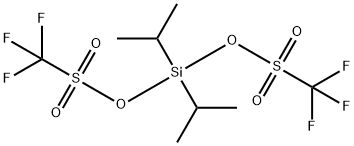
DIISOPROPYLSILYL BIS(TRIFLUOROMETHANESULFONATE)
- Product NameDIISOPROPYLSILYL BIS(TRIFLUOROMETHANESULFONATE)
- CAS85272-30-6
- CBNumberCB5678556
- MFC8H14F6O6S2Si
- MW412.4
- MDL NumberMFCD00043111
- MOL File85272-30-6.mol
- MSDS FileSDS
Chemical Properties
| Boiling point | 85-86 °C2 mm Hg(lit.) |
| Density | 1.396 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point | >230 °F |
| form | liquid |
| Specific Gravity | 1.396 |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| BRN | 3567500 |
| UNSPSC Code | 12352001 |
| NACRES | NA.22 |
Safety
| Symbol(GHS) |

|
| Signal word | Danger |
| Hazard statements | H314 |
| Precautionary statements | P280-P303+P361+P353-P304+P340+P310-P305+P351+P338-P363-P405 |
| Hazard Codes | C |
| Risk Statements | 34-37 |
| Safety Statements | 26-36/37/39-45 |
| RIDADR | UN 3265 8/PG 2 |
| WGK Germany | 3 |
| F | 10-21 |
| HazardClass | 8 |
| PackingGroup | III |