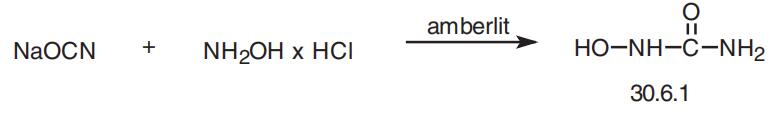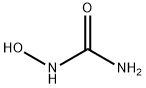
Hydroxyurea
- Product NameHydroxyurea
- CAS127-07-1
- CBNumberCB8249322
- MFCH4N2O2
- MW76.05
- EINECS204-821-7
- MDL NumberMFCD00007943
- MOL File127-07-1.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 135-140 °C |
| Boiling point | 136.04°C (rough estimate) |
| Density | 1.457±0.06 g/cm3(Predicted) |
| refractive index | 1.4840 (estimate) |
| storage temp. | 2-8°C |
| solubility | H2O: 50 mg/mL |
| form | powder |
| pka | 10.56±0.23(Predicted) |
| color | white |
| Odor | odorless or almost odorless |
| Water Solubility | soluble |
| Merck | 14,4848 |
| BRN | 1741548 |
| Stability | Stable for 2 years as supplied from date of purchase. Solutions in water may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 127-07-1(CAS DataBase Reference) |
| NCI Dictionary of Cancer Terms | Droxia; Hydrea; hydroxyurea |
| FDA UNII | X6Q56QN5QC |
| NCI Drug Dictionary | Droxia |
Safety
| Symbol(GHS) |

|
|||||||||
| Signal word | Danger | |||||||||
| Hazard statements | H340-H361 | |||||||||
| Precautionary statements | P201-P308+P313 | |||||||||
| Hazard Codes | T,Xn | |||||||||
| Risk Statements | 46-63-61-40 | |||||||||
| Safety Statements | 53-36/37-45-36-22 | |||||||||
| RIDADR | 2811 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | YT4900000 | |||||||||
| Hazard Note | Toxic | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29242100 | |||||||||
| Hazardous Substances Data | 127-07-1(Hazardous Substances Data) | |||||||||
| Toxicity | dog,LD50,intravenous,> 1gm/kg (1000mg/kg),Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 23, Pg. 682, 1992. | |||||||||
| NFPA 704: |
|