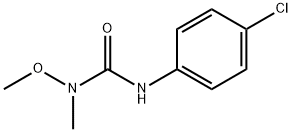
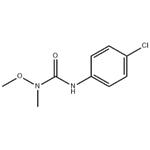
Uses
The certified reference material (CRM) is intended to be used as a calibrant for chromatography and other analytical techniques.
Monolinuron CRM may also be used as given below:
- Determination of 252 pesticides extracted from surface water samples by solid-phase extraction (SPE) using liquid chromatography quadrupole-orbitrap high-resolution tandem mass spectrometry
- Extraction of 13 pesticides from four groundwater and three river water samples using silica-based MSU-1 mesoporous solid as a sorbent for their solid-phase extraction (SPE) followed by ultra-performance liquid chromatography-triple quadrupole-mass spectrometric (UHPLC- QqQ-MS/MS) determination
- Development and validation of a UHPLC-diode array detection (DAD) method for simultaneous determination of urea and tebuthiuron herbicides in four fresh vegetable samples
- Multi-residue analysis of 50 herbicides in 51 grain samples of soybean, corn, and whe at by ultra-performance liquid chromatography-electrospray ionization-mass spectrometry (UPLC-ESI-MS)
- Extraction of 109 pesticide residues from different chemical classes from 345 tomato samples by modified QuEChERS method and their determination using liquid chromatography-tandem mass spectrometry (LC-MS/MS)
- Simultaneous analysis of 238 pesticides and 78 veterinary drugs in 80 bovine milk samples using ultra-fast liquid chromatography-tandem mass spectrometry (UFLC-MS/MS) and gas chromatography-tandem mass spectrometry (GC-MS/MS)
Definition
ChEBI: Monolinuron is a member of ureas.
Reactions
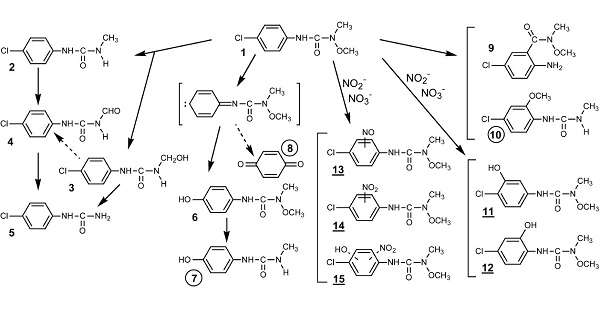
Nitrite or nitrate induced-photolysis of monolinuron (a phenyl urea herbicide) using the 300–450 nm light excitation gives rise to various photo-products. The direct photolysis of monolinuron proceeds via two main pathways yielding, respectively, 3-(4-chlorophenyl)-1-methylurea, which results from demethoxylation of the N-terminus substituted functional group and 3-(4-hydroxyphenyl)-1-methoxy-1- methylurea obtained by photohydrolysis of the C-Cl bond[1].
Degradation pathways of monolinuron by direct and nitrite (or nitrate) induced photolysis. Specific photoproducts: circled numbers (direct); underlined numbers (induced):
Safety Profile
Moderately toxic by
ingestion. Experimental teratogenic and
reproductive effects. When heated to
decomposition it emits very toxic fumes of
Cland NOx.
References
[1] S. Nélieu. “Nitrite and nitrate induced photodegradation of monolinuron in aqueous solution.” Environmental Chemistry Letters 2 2 (2004): 83–87.