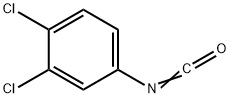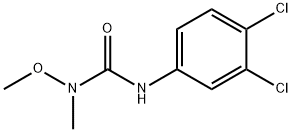
Linuron
- Product NameLinuron
- CAS330-55-2
- CBNumberCB2749714
- MFC9H10Cl2N2O2
- MW249.09
- EINECS206-356-5
- MDL NumberMFCD00055249
- MOL File330-55-2.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 93-94°C |
| Boiling point | 180-190°C |
| Density | 1.4909 (rough estimate) |
| refractive index | 1.6100 (estimate) |
| Flash point | 11 °C |
| storage temp. | APPROX 4°C |
| solubility | DMSO : 100 mg/mL (401.46 mM; Need ultrasonic) |
| pka | 12.13±0.70(Predicted) |
| form | Crystalline Solid |
| color | White |
| Water Solubility | 0.0075 g/100 mL |
| BRN | 2128725 |
| CAS DataBase Reference | 330-55-2(CAS DataBase Reference) |
| EWG's Food Scores | 6 |
| FDA UNII | 01XP1SU59O |
| Proposition 65 List | Linuron |
| NCI Drug Dictionary | Provigil |
| NIST Chemistry Reference | N'-(3,4-Dichlorophenyl)-N-methoxy-N-methylurea(330-55-2) |
Safety
| Symbol(GHS) |
  
|
|||||||||
| Signal word | Danger | |||||||||
| Hazard statements | H302-H351-H360FD-H373-H410 | |||||||||
| Precautionary statements | P202-P260-P264-P273-P301+P312-P308+P313 | |||||||||
| Hazard Codes | T;N,N,T,F | |||||||||
| Risk Statements | 61-22-40-48/22-50/53-62-39/23/24/25-23/24/25-11 | |||||||||
| Safety Statements | 53-45-60-61-36/37-16-7 | |||||||||
| RIDADR | UN 3077 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | YS9100000 | |||||||||
| HS Code | 29280000 | |||||||||
| Hazardous Substances Data | 330-55-2(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 orally in rats: 1500 mg/kg (Bailey, White) | |||||||||
| NFPA 704: |
|