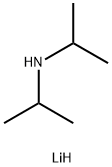
Chemical Properties
Lithium diisopropylamide (LDA) is a white pyrophoric powder. Freshly prepared, it is soluble in hydrocarbons (in hexane about 10 %), but it tends to precipitate irreversibly from solution as a polymer on heating or prolonged storage. In ethers the solubility is much higher, but with the exception of tetrahydropyran, LDA is decomposed at a rate depending on the ether, concentration, and temperature.
Lithium diisopropylamide can be conveniently prepared from butyllithium and diisopropylamine or purchased as a 2 mol/L (25 %) solution in THF and a mixture of various hydrocarbons. Although LDA is unstable in pure THF (at room temperature a 25% solution loses about 1% of its activity per day), the commercially available compositions containing only a limited amount of THF are satisfactory stable for technical applications.
Chemical Properties
dark yellow to orange or dark red-brown solution. Lithium Diisopropylamide (LDA) [4111-
54-0], LiN(CH(CH3)2)2, Mr 107.14 is a white
pyrophoric powder. Freshly prepared, it is soluble in hydrocarbons (in hexane about 10 %), but it
tends to precipitate irreversibly from solution as a
polymer on heating or prolonged storage. In
ethers the solubility is much higher, but with the
exception of tetrahydropyran, LDA is decomposed at a rate depending on the ether,
concentration, and temperature.
Uses
pH adjuster in colognes and toilet waters. In organic synthesis, particularly, the lithium salt.
Uses
Lithium diisopropylamide is a sterically hindered nonnucleophilic strong base used for selective deprotonations, especially for the production of kinetic enolates (i.e., the thermodynamically less favored isomer) and (hetero-)aromatic carbanions. In the production of the serum lipid regulating agent Gemfibrozil one key intermediate is formed by quenching an ester enolate with 1-bromo-3-chloropropane.
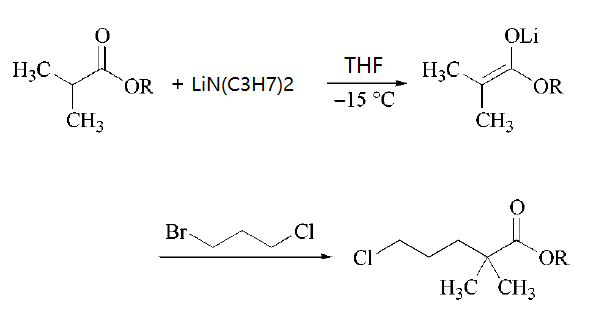
Uses
Lithium diisopropylamide solution (LDA) can be used:
- As an initiator in the anionic polymerization of D,L-lactide and methyl methacrylate.
- To facilitate ester enolization.
- To convert carboxylic acids to enediolate intermediates for preparing trifluoromethyl ketones.
- In ortho-lithiation of arylsulfonyloxazolidinones to prepare N-substituted saccharin analogs.
- To catalyze ortholithiation and Fries rearrangement of aryl carbamates.
- As a promoter in the isomerization of allylic ethers to (Z)-propenyl ethers.
Synthesis
Lithium diisopropylamide has been synthesized by two classical routes. Th 1ost widely adopted route involves in situ formation by deprotonation of diisopropylamineby an alkyllithium reagent, such as butyllithium.Addition of a solutionof butyllithium (in hexane) to a stirred solution of diisopropylamine (freshly distilledfrom CaCl) in tetrahydrofuran directly gives the required solution of lithium diisopropylamide in tetrahydrofuran. This deprotonation process has been reported to be efficient over a wide temperature range (from -78°C to 25°C). The resulting solution of lithium diisopropylamide in tetrahydrofuran appears to be stable, and consequently canbe stored at room temperature for a short period without loss of basicity.
Lithium diisopropylamide can also be synthesized directly by addition of lithium todiisopropylamine.This approach gives better quality crystalline lithium diisopropylamide, especially if required for single-crystal X-raystructure determination, than the butyllithium route.The synthesis of lithium diiso-propylamide by in situ deprotonation of diisopropylamine in tetrahydrofuran by analkyllithium reagent is, however, the most commonly used method.Butyllithium isusually the alkyllithium of choice, but otheralkyllithiums have been used (e.g., MeLi)
General Description
This material is in a solution of THF/Hexanes (ca. 1:7 ratio, respectively)
Flammability and Explosibility
Spontaneously flammable in air (pyrophoric)
Purification Methods
It is purified by refluxing over Na wire or NaH for 30minutes and then distilled into a receiver under N2. Because of the low boiling point of the amine, a dispersion of NaH in mineral oil can be used directly in this purification without prior removal of the oil. It is HIGHLY FLAMMABLE, and is decomposed by air and moisture. [Wittig & Hesse Org Synth 50 69 1970, Beilstein 4 H 154, 4 I 369, 4 II 630, 4 III 274, 4 IV 510.]