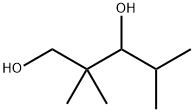
Chemical Properties
(96% pure): White solid. Slightly soluble in water; soluble in alcohol,
acetone, ether, and benzene. Combustible.
Chemical Properties
2,2,4-Trimethyl-1,3-pentanediol is a somewhat volatile liquid.
Uses
Main uses of 2,2,4-Trimethyl-1,3-pentanediol are as a
component of polyester resins used in food packaging and as an insect repellent. Industrial exposure is likely to be by
direct contact with the liquid. Vapors or mists may be
encountered under some conditions.
Uses
Polyester resins, plasticizers, lubricants, surface
coatings and printing inks, insect repellent.
Uses
2,2,4-Trimethyl-1,3-pentanediol is used in the synthesis of monoisobutyrate derivatives used in film forming auxilleries in paint substances.
Production Methods
2,2,4-Trimethyl-1,3-pentanediol is made by reacting isobutyryl
chloride with ethyl isobutyrate.
Definition
2,2,4-Trimethyl-1,3-pentanediol (TMPD) glycol, with one primary and one secondary hydroxyl function, shows the typical reactivity of a diol. In comparison to other diols, such as 1,4-cyclohexanedimethanol) or NPG (Neopentyl glycol), TMPD glycol has an unsymmetrical structure and shielded hydroxyl groups.
Flammability and Explosibility
Non flammable
Toxicology
2,2,4-trimethyl-1,3-pentanediol (TMPD Glycol) is classified as "slightly toxic". In rabbits, moderate eye irritation was observed. Slight to no skin irritation occurred in guinea pigs, and no skin sensitization was found. Repeated skin application studies in humans gave no evidence of irritation, sensitization, photosensitization, or systemic toxic effects. In humans, TMPD glycol is rapidly excreted in the urine, partly unchanged, partly as the glucuronide and sulfate conjugates, and partly as 2,2,4-trimethyl-3-hydroxyvaleric acid. LD50 2000 (rat, oral), LD50 145 (rat, intravenous).