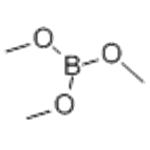
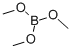Chemical Properties
Water-white moisture-sensitive liquid, fumes in the air. It forms an azeotrope with methanol with an azeotrope of 55°C. Miscible with ether, methanol, hexane, tetrahydrofuran; It is stable in anhydrous state and decomposes into methanol and boric acid in presence of water.

Uses
Trimethyl borate is a useful reagent in organic synthesis. It is involved in the production of resins, waxes and paints and acts as a methylation agent. As a boron source, Trimethyl borate is used to prepare flame retardants, anti-oxidants and corrosion inhibitors. It reacts with Grignard reagents followed by hydrolysis to prepare boronic acid. It is also used as a precursor of borate esters, which finds application in the Suzuki coupling reaction. It is used as neutron detector gas in the presence of a scintillation counter; as a promoter of diborane reactions.
Preparation
The preparation method of trimethyl borate
1. The direct reaction between boric acid and methanol is as follows:
3CH30H+H3B03→B (OCH3) 3+3H20
Usually the trimethyl borate formed in the reaction forms an azeotrope with excess methanol and is distilled out together, and then the trimethyl borate is obtained by separating the azeotrope.
2. The direct reaction between boron oxide and methanol is as follows:
B203+6CH30H→2B (OCH3) 3+3H20
3. Borax, methanol and sulfuric acid are directly reacted, and the reaction formula is :
Na2B4O7.1OH2O+12CH30H+2H2S04→4B (OCH3)3+2NaHS04+17H20
Application
Trimethyl borate reacts with a Grignard reagent or organolithium compounds to yield dimethyl boronates, which upon subsequent aqueous acid treatment afford corresponding boronic acids. The resultant boronic acids or esters are useful intermediates in various cross-coupling reactions such as Suzuki coupling and Chan-Lam coupling. It is also used in the preparation of sodium borohydride.
Definition
ChEBI: Trimethyl borate is a member of the class of borate esters obtained by the formal condensation of three equivalents of methanol with boric acid.
Reactions
Trimethyl borate B(OCH3)3 is a popular borate ester used in organic synthesis.
borate esters are prepared from alkylation of trimethyl borate:
ArMgBr + B(OCH3 )3 → MgBrOCH3 + ArB(OCH3 )2
ArB(OCH3 )2 + 2H2O → ArB(OH)2 + 2 HOCH3
General Description
Trimethyl borate appears as a water-white liquid. Denser than water. Vapors heavier than air. Used as a solvent and fungicide for fruit.
Air & Water Reactions
Highly flammable. Rapidly decomposes in water.
Reactivity Profile
Borates, such as Trimethyl borate, behave similarly to esters in that they react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters/borates with alkali metals and hydrides.
Health Hazard
Trimethyl borate may cause toxic effects if inhaled or absorbed through skin. Inhalation or contact with material may irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Trimethyl borate will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
reaction suitability
reagent type: reductant
Safety Profile
Moderately toxic by
ingestion, skin contact, and intraperitoneal
routes. An eye irritant. A very dangerous fire
hazard when exposed to heat, flame, or
oxidizers. Moderately explosive when
exposed to flame. Will react with water or
steam to produce toxic and flammable
vapors. To fight fire, use dry chemical, CO2,
spray, foam. When heated to decomposition
it emits acrid smoke and irritating fumes.
See also ESTERS and BORON
COMPOUNDS.
Purification Methods
Carefully fractionate the borate through a gauze-packed column. Re-distil and collect it in weighed glass vials and seal them. Keep it away from moisture. It undergoes alkyl exchange with alcohols and forms azeotropes, e.g. with MeOH the azeotrope consists of 70% (MeO)3B and 30% MeOH with b 52-54o/760mm, d 0.87. [Charnley et al. J Chem Soc 2288 1952, Gerrard & Lappert Chem Ind (London) 53 1952, Schlesinger et al. J Am Chem Soc 75 213 1953.] It has also been dried with Na and then distilled. [Beilstein 1 IV 1269.]
Toxics Screening Level
The initial threshold screening level (ITSL) for trimethyl borate is 770 μg/m3 (1-hour averaging time).