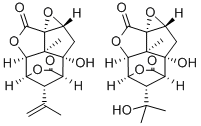
Description
Picrotoxin, also known as cocculin, appears as white to light beige crystalline material. It is
isolated from Cocculus indicus (Fructus cocculi), fishberries, or Indian berries. Picrotoxin is a
colourless, flexible, shining, prismatic crystals, or a micro-crystalline powder. It is odourless,
has a very bitter taste, and is permanent in the air. Picrotoxin is soluble in hot water,
readily soluble in strong ammonia water, and in aqueous solutions of sodium hydroxide,
soluble in dilute acids, and alkalis as well as in glacial acetic acid, sparingly soluble in
chloroform and very slightly soluble in cold water and alcohol. Picrotoxin is stable under
normal temperatures and pressures and decompose when exposed to light. Picrotoxin is
incompatible with strong oxidising agents, strong acids, strong bases, light.
Chemical Properties
Picrotoxin is an odorless crystalline solid with a very bitter taste.
Chemical Properties
white to off-white powder or crystals
Physical properties
Appearance: colorless, soft, and glossy rhombic pillar-shaped crystal or crystalline
fine powder. It is odorless and tastes very bitter. It is steady in the air, but it metamorphoses easily when exposed to light. Solubility: its solubility in water is 0.3%;
soluble in diluted acid; slightly soluble in ether or chloroform.
History
The seeds of Menispermaceae are traditionally used as fishing bait or pesticide, as
well as pediculicide . Once it was used for brewing beer to impart a more intoxicating quality to beer in the nineteenth century . In 1812, cocculin was firstly
isolated by Pierre Boullay and named picrotoxin, a combination of the Greek words
“picros” and “toxicon.” Picrotoxin showed strong physiological action and was
once extensively studied.
The action mechanism of picrotoxin is a controversial issue. Newland et?al. considered that picrotoxin enhanced the occurrence of a desensitized state or an allosterically blocked state of GABAA receptor , while Jong et?al. showed that picrotoxin
acts as a noncompetitive channel blocker for the GABAA receptor chloride channels, rather than a receptor antagonist .
Uses
Picrotoxin has been used:
- as non-competitive antagonist for the γ-aminobutyric acid (GABAA) for studying synchronized burst (SB) dynamics
- as a component of extracellular saline for blocking glutamate and acetylcholine receptors in neurons
- as a component of artificial cerebrospinal fluid (ACSF) for voltage-clamp recordings studies in dissociated cortical neurons
General Description
A poisonous berry, the dried fruit of Anamirta PICROTOXIN L. Contains several substances including about one percent picrotoxin. Pure picrotoxin occurs as shiny leaflets with an intensely bitter taste or as a microcrystalline powder. Very poisonous!. Used in medicine as a central nervous system stimulant and antidote for barbiturate poisoning. Not currently regarded as a useful therapeutic agent.
Reactivity Profile
PICROTOXIN contains picrotoxin, a molecular compound of picrotoxinin and picrotin. May react with strong oxidizing agents and with strong reducing agents.
Health Hazard
Highly toxic and a dose of 20 mg may produce symptoms of severe poisoning. A human lethal dose of 1.5 mg/kg has been reported. It is an alkaloid convulsant poison.
Fire Hazard
When heated to decomposition PICROTOXIN emits acrid smoke and fumes. Avoid decomposing heat.
Biological Activity
GABA A receptor antagonist; potent CNS stimulant.
Biochem/physiol Actions
Picrotoxin is a competitive inhibitor of glycine receptors (GlyRs). It is a non-competitive antagonist, which binds to ligand bounded γ-aminobutyric acid (GABAA) receptor.
Pharmacology
The pharmacological effects of picrotoxin are focused on the central nervous system and GABA receptor. Han et?al. showed that picrotoxin significantly inhibited
the nociceptive discharges of parafascicular neurons of the thalamus in rabbit . It
was also showed that picrotoxin, as a GABA receptor antagonist, could enhance the
effects of electroanalgesia. These results provided theoretical basis for algogenesis
and pain management . Xu et?al. showed that GABA receptor might be involved
in decreased blood pressure induced by hypoxia in rabbits and picrotoxin could
block the decreased blood pressure induced by hypoxia . This result provided
strong evidence for the treatment of decreased blood pressure induced by hypoxia
using picrotoxin. Picrotoxin also showed direct effects on the heart. It inhibits
cardiac-specific conduction system and decreases heart rate and cardiac contractility, as well as cardiac action potential . Picrotoxin mainly activates the mesencephalon and oblongata as a GABA receptor antagonist, and high dosage of
picrotoxin may also activate the spinal cord and cerebrum.
Clinical Use
Acute barbital poisoning is very common and may be life-threatening. Many studies
reported the superior effects of picrotoxin in rescuing barbital poisoning .
Picrotoxin can be used in serious barbital poisoning patients when conventional
central nervous system stimulants are ineffective. The dosage and route of administration depend on the severity and state of the illness. It was usually administrated
intramuscularly or intravenously (3–9?mg), once every 15–30?min, until corneal and
deglutition reflex or slight tremble in the face and four limbs appears.
Safety Profile
A human poison by
ingestion. Poison experimentally by most
routes. Human systemic effects by ingestion:
somnolence, gastrointestinal effects. An
alkaloid convulsant poison. When heated to
decomposition it emits acrid smoke and
irritating fumes.
Potential Exposure
An alkaloid poison and convulsant. Used in medicine as a CNS stimulant and antidote for barbiturate poisoning. Reportedly, this material is not currently regarded as a useful therapeutic agent since it is not a selective respiratory stimulant.
Shipping
UN3462 Toxins, extracted from living sources, solid, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN3172 Toxins, extracted from living sources, (solid or liquid) Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Purification Methods
Crystallise picrotoxin from H2O or Me2CO/H2O. The monoacetate has m 244-245o (*C6H6). [Meyer & Bruger Chem Ber 31 2958 1898, Johns et al. J Chem Soc 4717 1956, Beilstein 19 III/IV 5245.]
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Waste Disposal
It is inappropriate and possibly dangerous to the environment to dispose of expired or waste drugs and pharmaceuticals by flushing them down the toilet or discarding them to the trash. Household quantities of expired or waste pharmaceuticals may be mixed with wet cat litter or coffee grounds, doublebagged in plastic, discard in trash. Larger quantities shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator.