Reactions
Non-pyrophoric, air-stable derivative suitable as a replacement for the neat phosphine in a variety of stoichiometric and catalytic processes.
Chemical Properties
White powder
Uses
Tricyclohexylphosphine tetrafluoroborate may be used in the following processes:
- As a ligand for preparing C-homoaporphine alkaloids via microwave-assisted direct-arylation.
- To synthesize poly-[9,9-bis(3-propylamide-2-methylpropyl sulfonic acid) fluorene]-co-(4,4′-diphenyl) (PFDBSO3H), which can be employed as a template and doping agent for enhancing the conductivity of poly(3,4-ethylenedioxythiophene) (PEDOT) films.
- To improve the reactivity of palladium-catalyzed Suzuki-Miyaura cross-coupling reaction between MIDA boronates and less activated alkenyl tosylates.
Uses
Tricyclohexylphosphonium Tetrafluoroborate acts as a reagent in the synthesis of potent JAK2 inhibitor BMS-911543 as a potential treatment for myeloproliferative disorders, preparation of Lapatinib intermediate via arylation of furfural with bromochlorofluorobenzyloxyphenylquinazolinamine, structure-activity relationships of carboline and carbazole derivatives as ATP-competitive kinesin spindle protein inhibitors.
General Description
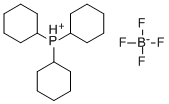
Tricyclohexylphosphine tetrafluoroborate (PCy
3·HBF
4) is an inexpensive and air-stable phosphine ligand, commonly used in cross-coupling reactions.
reaction suitability
reaction type: Arylations
reaction type: Cross Couplings
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling