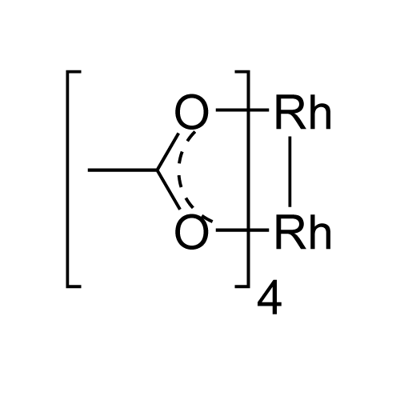
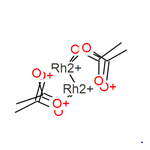
Chemical Properties
Rhodium(II) acetate dimer is Emerald Green Powder. Soluble in methanol. It is soluble in water, while the relative solubility of copper acetate in water is small.

Uses
Rhodium (II) Acetate Dimer is a effective catalyst for ylide formation and it is also catalyst for cyclopropanation of alkenes, oxidation of alcohols, cyclization reactions involving -diazo carbonyl groups, insertion into C-H and X-H bonds (X-H is NH or SH or OH). More reactive and useful in differentiating ribonucleosides and deoxynucleosides. It is also used in functionalizing fullerenes into polymers. Efficient catalyst for hydrogen transfer from 2-propanol to cyclohexanone and other unsaturated compounds.
Application
Rhodium (II) Acetate Dimer is used in the preparation of molybdenum triisopropylbenzoate isonicotinate which maintains ambivalent properties.
Preparation
Rhodium(II) acetate dimer synthesis: A suspension of 10.0g. of Rh(OH)3.H20 in 400 ml. of glacial acetic acid dissolved upon refluxing for 18 hr. to give a deep emerald-green solution. Most of the acetic acid was evaporated on a steam bath; remaining traces mere removed by heating the residue at 120° for 1hr. The residue was then extracted with boiling acetone until the extract was colorless rather than bluish green. The extract was quickly passed through a fritted glass filter, concentrated on a steam bath to 1/3 its original volume, and placed in the freezing compartment of a refrigerator for 18hr. The resulting large dark green crystals were collected on a filter, washed with small portions of ice-cold acetone, and dried at 110°. When first collected, the product is the weak adduct [Rh(OOCCH3)2(CH3)2C0]2; however, acetone is lost fairly readily at room temperature and immediately in the drying oven; yield, 6.2g. (48%).
Reactions
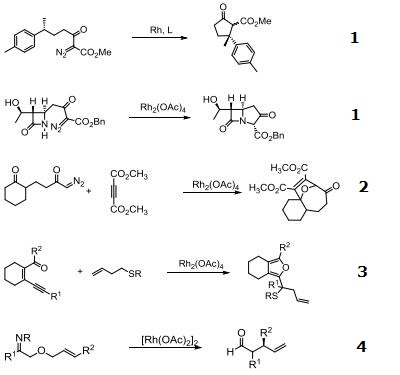
Catalyst for insertion into C-H and X-H bonds.
Catalyst for Ylide generation.
Doyle Kirmse Reaction of Allylic Sulfides with Diazoalkane.
Claisen rearrangement.
Epoxides from aldehydes.
Synthesis of aziridines from allylic N-tosyloxycarbamates.
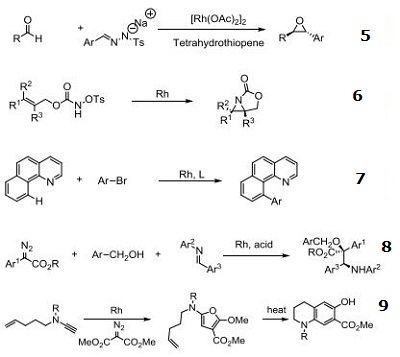
Rh/NHC catalyzed direct intermolecular arylation of C-H bonds.
Chiral Bronsted acid-Rh catalyzed three component reactions of diazo compounds with alcohols and imines.
Rh-catalyzed cyclopropenations of ynamides.
Tandem asymmetric aza-Darzens/ring-opening reactions.
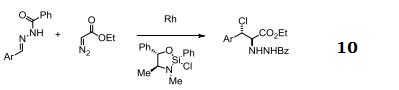
reaction suitability
reaction type: C-H Activation
reagent type: catalyst
Purification Methods
Dissolve 5g of the salt in boiling MeOH (ca 600mL) and filter. Concentrate it to 400mL and chill overnight at ca 0o to give dark green crystals of the MeOH adduct. Concentration of the mother liquors gives a further crop of [Rh(OAc)2]2.2MeOH. The adduct is then heated at 45o in a vacuum for 2hours (all MeOH is lost) to leave the emerald green crystals of the actetate. [Legzdins et al. J Chem Soc (A) 3322 1970.] Alternatively dissolve the acetate in glacial AcOH and reflux for a few hours to give an emerald green solution. Evaporate most of the AcOH on a steam bath, then heat the residue at 120o/1hour. Extract the residue with boiling Me2CO. Filter, concentrate to half its volume and keep at 0o/18hours. Collect the crystals, wash them with ice cold Me2CO and dry them at 110o. It is soluble in most organic solvents with which it forms adducts including Me3N and Me2S and gives solutions with different colours varying from green to orange and red. [UV: Johnson et al. Inorg Chem 2 960 1963, Beilstein 1 H 124.]