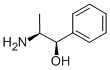
Description
Phenylpropanolamine (PPA) is a mixed-acting sympathomimetic
amine similar to ephedrine. Its primary mechanism of
action is through direct a-adrenergic agonism, but there is also
indirect stimulation of norepinephrine release. In November
2000, the US Food and Drug Administration (FDA) Nonprescription
Drugs Advisory Committee determined that there is
a significant association between PPA and hemorrhagic stroke
and recommended that PPA not be considered safe for over-thecounter
use. At this time, a letter was issued to manufacturers
requesting voluntary removal of PPA from their products. Later,
in 2005, the FDA published a Tentative Final Monograph for
PPA-containing products proposing Category II status (not
generally recognized as safe and effective). To date, no Final
Monograph has been released; however, all manufacturers have
removed PPA from their products.
Originator
Propadrine, MSD ,US ,1941
Uses
PPA was used as a nasal decongestant and as an anorectic.
Studies have shown benefit in humans for urinary incontinence,
and PPA is still used in veterinary medicine for this purpose.
Uses
The central nervous excitement
Uses
Norephedrine, also called Phenylpropanolamine (PPA), is a synthetic form of the ephedrine alkaloid. Used in treatment of urinary incontinence in dogs and cats; decongestant nasal. After reports of the occurrence of intracranial hemorrhage and other adverse effects, including several deaths, PPA is no longer sold in USA and Canada.
Definition
ChEBI: (-)-norephedrine is an amphetamine that is propylbenzene substituted by a hydroxy group at position 1 and by an amino group at position 2 (the 1R,2S-stereoisomer). It is a plant alkaloid. It has a role as a plant metabolite. It is a member of amphetamines and a phenethylamine alkaloid.
Manufacturing Process
In one route as described in US Patent 2,151,517, 10.7 kg of technical benzaldehyde is vigorously agitated with a solution of 11.0 kg of sodium bisulfite in 50.0 liters of water until the formation of the addition-product is complete. Simultaneously, 8.25 kg of nitroethane is dissolved in a solution of 4.5 kg of caustic soda in 20.0 liters of water and the resultant warm solution is added with vigorous stirring to the magma of benzaldehyde sodium bisulfite. The mixture is agitated for 30 minutes and then allowed to stand overnight.
The aqueous portion of the mixture is now siphoned off from the supernatant layer of oily phenylnitropropanol and replaced with a fresh solution of 11.0 kg of sodium bisulfite in 50.0 liters of water. The mixture of phenylnitropropanol and bisulfite solution is now vigorously agitated for 15 minutes in order to remove and recover small amounts of unreacted benzaldehyde, and is then again allowed to stratify. This time, the phenylnitropropanol is siphoned off and filtered to remove a small amount of resinous material. The aqueous solution of sodium bisulfite remaining behind is reacted with benzaldehyde, as described above, thus making the process continuous.
The 1-phenyl-2-nitropropanol thus obtained is a colorless oil, specific gravity 1.14 at 20°C, odorless when pure, volatile with steam and boiling at 150° to 165°C under a pressure of 5 mm of mercury. It is soluble in alcohol, ether, acetone, chloroform, carbon tetrachloride, benzene and glacial acetic acid. The yield of 1-phenyl-2-nitropropanolobtained by this procedure is 17.1 to 17.7 kg.
It is hydrogenated and converted to the hydrochloride in subsequent steps. The hydrogen chloride has a melting point of 192°-194°C.
In an alternative route described in US Patent 3,028,429 propiophenone may be reacted with an alkyl nitrite to give isonitrosopropiophenone which is then hydrogenated and finally converted to the hydrochloride.
brand name
A.g.multix;Acutrim;Adistop-f;Amertuss;Amplisiex;Am-tuss liq;Anorexin;Antiadipositum;Apoephedrin;e
Aridose;Arm;Bifed-20;Biphetane;Biphetap;Blu-hist;Brocon cr;Bromanate;Bromepaph;Brometapp;Bronco-quintoxil;Cenadex;Chlor-rest;Cinturex;Cletanol;Codimal;Cofpac;Cold cap;Coldecon;Col-decon;Conex-grippe;Contop;Coricidin f;Corsym;Coryztime;Cremacoat;Dalca;Decidex;Decomine;Demazine;Deprecstop;Dexatrim;D-sinus;Efed ii;Eficol;Endal;Endecon;Endex;E-son;Espornade spansule;E-tapp 3;Exyphen;Factus;Fornagest;Fugoa n;Gardax;Ginsopan;Headway;Histade;Histatapp;Hsp 540;Ipercron;Kol-tac;Kontexin;Koryza;Leder;Lipo-sinahist;Lunerin;Mardram;Minus-x;Monatuss;Mucolyt-expecto;Nd-hist;Nectatussin;Neosoldana;Nexaam;Nobese;Norephedrine;Nornatane;Ornacol;Ornatos;Ornex;Pabron nose;Panacorn;Panadyl;Parhist;Partapp;Pholcolix spansule;Pneumidex;Polcimut;Profenade;Propagest;Reduzin;Rhindecon;Rhinergal;Rhinervert;Rhinicept;Rhinidrin;Rhinocap;Rinexin;Rinomar;Rinotussal;Rinurel lictus;Rinurel tablets;Rinutan;Rotabromophen;Rupton;Rynatapp;Rynex;Ryza-gesic;Sacietyl;Scotuss;Secron;Sinacin;Sinudan;Sinu-lets;Sinutab cough l;Spandecon;Srda;Sto-caps;Sulfa-probocon;Symptrol;Taviset;Tinaroc;Totolin;Tricon;Tri-congestic;Triogesic elixir;Triominic;Triotussic;Turbispan;Tussilene-dm;V cold;Veltane;Veltap;Vernate;Vistaminic;Voxin-pg;W 58;X 112 antiadipo;Zerinol;Partuss;Permatrim;Phenapap.
Therapeutic Function
Nasal decongestant, Anorexic
World Health Organization (WHO)
Phenylpropanolamine, a symopathomimetic amine, has been
widely available in over-the-counter preparations since 1941. It is one of the most
frequently used nasal decongestants and it is a common ingredient in preparations
for weight reduction, although doubts have been raised about its usefulness in this
indication. It is also used in stress incontinence. Its use has been associated with
occasional excessive elevation of blood pressure, especially in hypersensitive
individuals.
General Description
Phenylpropanolamine is the N-desmethylanalog of ephedrine and thus has many similar properties.Lacking the N-methyl group, phenylpropanolamine is slightlymore polar, and therefore does not enter the CNS as well asephedrine. This modification gives an agent that has slightlyhigher vasopressive action and lower central stimulatoryaction than ephedrine. Its action as a nasal decongestant ismore prolonged than that of ephedrine. It is orally active.Phenylpropanolamine was a common active component inOTC appetite suppressants and cough and cold medicationsuntil 2001 when the Food and Drug Administration (FDA)recommended its removal from such medications, becausestudies showed an increased risk of hemorrhagic stroke inyoung women who took the drug.
Safety Profile
Moderately toxic by subcutaneousroute. When heated to decomposition it emits very toxicfumes of NOx.
Toxicity evaluation
The primary action of PPA is direct a-adrenergic agonism,
though it also causes indirect release of norepinephrine at
postganglionic sympathetic nerve terminals. PPA also has
weak β agonistic properties. Hypertension results from α adrenergic
mediated vasoconstriction of peripheral blood
vessels. Reflex bradycardia is common. Sympathomimetic
effects can produce anxiety, insomnia, agitation, tremor,
tachycardia, and mydriasis.